What molecule am I?
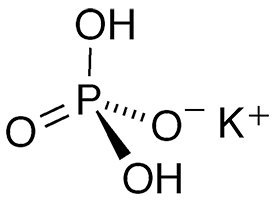

Potassium dihydrogen phosphate (KH2PO4) is an inorganic salt with multiple uses. It is also known as monopotassium phosphate, potassium phosphate monobasic, potassium biphosphate, and potassium acid phosphate.
In US Patent 374,201 (1887), Carl. V. Petraeus of Camden, NJ, described a process for making potassium phosphates, specifically KH2PO4 with a slight excess of potassium. His starting materials were “bone, bone-black, South Carolina rock, or any compounds mainly composed of lime”. Petraeus’s somewhat convoluted process consisted of five steps:
- decomposing the starting material with sulfuric or oxalic acid;
- leaching with water to produce an impure “acid phosphate of lime” (calcium dihydrogen phosphate);
- treating the calcium dihydrogen phosphate with potassium sulfate;
- adding sufficient potassium carbonate or hydroxide to form a solution of KH2PO4 with excess potassium; and
- after filtering to remove the calcium salts, evaporating the solution to the point of crystallization.
Today, KH2PO4 is manufactured simply via the reaction between widely available phosphoric acid and potassium carbonate.
The uses of KH2PO4 include agricultural plant nutrients, buffering agents in pharmaceuticals and other preparations, and applications that take advantage of its nonlinear optical properties. To that end, in 2006 Changshui Fang and colleagues at Shandong University (Jinan, China) reviewed how lasers induce damage in large-aperture KH2PO4 crystals and suggested ways to improve the crystals’ optical properties.
Large-aperture crystals are useful in Pockels cells, in which an external electric field is used to control the cell’s refractive index, and in frequency-doubling devices used in inertial-confinement fusion power plants.
Potassium dihydrogen phosphate hazard information
| Hazard class* | GHS code and hazard statement |
|---|---|
| Not a hazardous substance or mixture |
*Globally Harmonized System (GHS) of Classification and Labeling of Chemicals.
Molecule in the News
Dichlromethane1 (methylene chloride, CH2Cl2), the Molecule of the Week for March 4, 2019, is a widely used solvent that has come under increasing pressure from health and environmental regulatory agencies in the past several years.
In the latest move this April, the US Environmental Protection Agency proposed a ban on dichloromethane for all consumer and most industrial and commercial uses, including adhesives, paint strippers, and degreasers. Dichloromethane is the second chemical to be reviewed under the 2016 revision of the Toxic Substances Control Act. The first was asbestos2, and the third is expected to be tetrachloroethylene3.
1. CAS Reg. No. 75-09-2.
2. CAS Reg. No. 1332-21-4.
3. CAS Reg. No. 127-18-4.
Molecule in the News
MOTW highlights molecules that appear in major news outlets. See this week's edition below.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
Potassium dihydrogen phosphate fast facts
| CAS Reg. No. | 7778-77-0 |
| SciFindern name | Phosphoric acid, potassium salt (1:1) |
| Empirical formula | H2O4KP |
| Molar mass | 136.09 g/mol |
| Appearance | Colorless crystals or white powder |
| Melting point | 253 °C |
| Water solubility | 226 g/L (20 °C) |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

