Hot Peppers: Muy Caliente
By Brian Rohrig December 2013

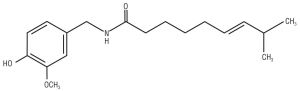
The “hot” in hot peppers is due to capsaicin (C18H27NO3), a colorless, odorless oil-like compound found in the fruit of a plant that is a close relative of the tomato. Capsaicin is primarily found in the membrane that holds the seeds. These plants are found in the Americas and were brought to Europe by explorer Christopher Columbus who mistakenly thought they were a relative of the black pepper (the plant we get pepper from). To distinguish them from the black pepper plant, hot peppers are usually called chili peppers, or just plain chilis in many parts of the world. Capsaicin is also found, in smaller amounts, in other spices, such as oregano, cinnamon, and cilantro.
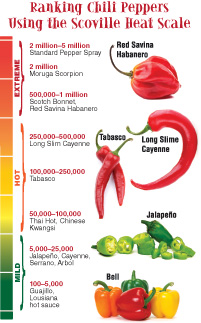
The hotness of a pepper is measured by the Scoville heat scale (below), which is a series of “heat units” that range from 0 to 16 million, depending on the capsaicin content of a pepper. Pure capsaicin tops the scale at 16 million heat units, and bell peppers rank at 0, since they contain no capsaicin. The Moruga Scorpion comes in at around 2 million heat units, the same capsaicin concentration as pepper spray! The jalapeno pepper ranges from 5,000 to 50,000 heat units, while the habanero ranks from 100,000 to 350,000 heat units.
Today, more sophisticated methods are used to determine how much capsaicin is in peppers, using instruments which measure concentrations in parts per million (ppm). One ppm of capsaicin means that 1 milligram of capsaicin is present in 1 kilogram of pepper. It is like having one red marble (one capsaicin molecule) in a bucket along with 999,999 white marbles (other molecules present in pepper). Capsaicin is so potent that even a concentration of 10 ppm would produce a long-lasting burning sensation on the tongue.
A large dose of capsaicin, in its concentrated form, could be toxic if ingested, yet the amount found in hot peppers is so small there is little risk of harm from the toxic effects of capsaicin itself. When dealing with it in its pure form, you must wear gloves and a respirator.
Scoville Heat Scale
The Scoville heat scale was devised in 1912 by the American pharmacist Wilbur Scoville. To test the hotness of pepper, Scoville would take an extract from a pepper and determine how much sugar water was required to dilute it before its “heat” could no longer be detected by a panel of volunteer taste-testers.
For example, if he had 1 milliliter (ml) of pepper extract, and it took 100 ml of sugar water to dilute it until its hotness was no longer detectable, then it would rank at 100 Scoville heat units. If it took 1,000 ml of sugar water to dilute 1 ml of extract, then it would rate 1,000 Scoville heat units.
Water or Milk?
If you find yourself eating spicy chicken wings that are hotter than you expected, what do you do? You take a big gulp of water, right? Actually, that would be a bad move. Water only makes it worse, similar to throwing water on a grease fire.
If you look at the structure of capsaicin (below), you will notice that one end of the molecule is made of a long hydrocarbon tail. Hydrocarbons are molecules made of hydrogen and carbon, and many common fuels, such as gasoline and candle wax, are derived from hydrocarbons.
Hydrocarbons tend to be nonpolar, meaning that, in the molecule, the negatively charged electrons and the positively charged protons are evenly distributed throughout. A polar molecule, on the other hand, has distinct regions of positive and negative charge—the shared electrons will tend to stay near the atom with the higher electronegativity, or greater ability to attract electrons. This side of the molecule will develop a partial negative charge while the other side develops a partial positive charge. The reason these charges are partial is because the bond is still covalent and the electrons are still being shared; they are just shared unequally. Any molecule that has a partial positive charge and a partial negative charge is called a polar molecule.
Water is a good example of a polar molecule because its individual bond polarities do not cancel, leaving the oxygen side of water with a partial negative charge and the hydrogen side with a partial positive charge
In the case of the capsaicin molecule, the individual bond polarities are arranged in such a way that they cancel each other out. The capsaicin molecule ends up being nonpolar, overall, because of its molecular structure, especially the long nonpolar hydrocarbon tail.
Polar substances tend to dissolve in other polar substances, while nonpolar substances tend to dissolve in other nonpolar substances. This tendency is summed up by the principle “like dissolves like.” When you drink water after eating a hot pepper, the water just spreads it around your mouth, making the pain worse.
Can you tell which pepper is hotter?
To determine how hot a pepper is, look at the stem that holds it to the plant. In general, the thinner the stem, the hotter the pepper. Some gardeners claim that if the stem is bent it will be hotter than if it is straight. If you look at peppers of the same species, small peppers tend to be hotter than larger peppers. Since peppers get hotter as they ripen, a red pepper will be hotter than a green one. Also, dried peppers will always be hotter than fresh peppers, because as water evaporates from the fruit, the amount of capsaicin remaining will be of a higher concentration.

Drinking milk or eating ice cream is the preferred solution because milk and ice cream contain molecules that are nonpolar, called casein. Casein molecules attract capsaicin molecules. They surround the capsaicin molecules and wash them away, in the same way that soap washes away grease. This explains why milk and ice cream can remove capsaicin molecules from your tongue. Casein forms the curds in sour milk. So, cottage cheese, which is primarily casein, would be great to ease the pain from eating chili peppers. A piece of bread or other starchy food, which is made of nonpolar molecules, would also help ease the discomfort.
I had an experience once while traveling in Louisiana that I will never forget. I bought a hot pickle at a gas station. I didn’t open it until I was driving on the highway. After one bite, my mouth felt like it was on fire. It seemed like an eternity until I found a convenience store, where I purchased a snack cake and shoved it into my mouth to alleviate the pain. It helped some, but the pain persisted for quite some time afterward. I have avoided most hot foods since then, especially hot pickles!
A Burning Sensation
When you eat a hot pepper, it definitely feels like your mouth is on fire. But if you were to stick a thermometer in your mouth, it would not register an increase in temperature. Believe it or not, even the hottest peppers do not really get hot. They trigger pain receptors in your tongue, mouth and back of your throat that send a signal to the brain, which is interpreted as heat. Since capsaicin is an irritant, this feeling of heat is the body’s way of compelling you to take some food or drink in an effort to remove the irritant.
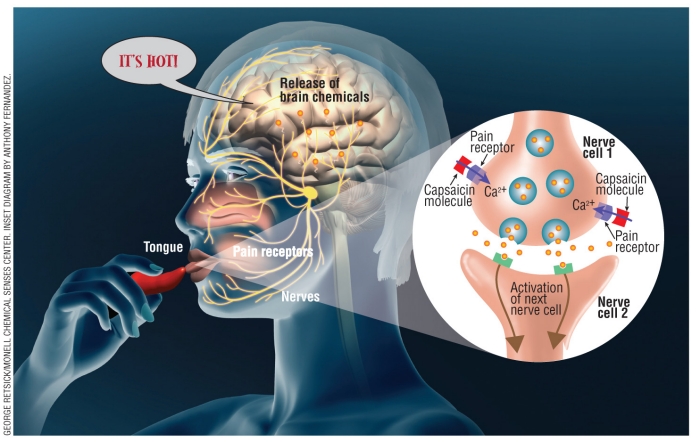
Figure 2. When a person eats a hot pepper, capsaicin molecules binds to pain receptors present on the surface of the tongue. These receptors send a signal to the brain that tells the person that the pepper is hot. This signal is relayed by successive neurons, each releasing brain chemicals that give that “hot” sensation. (Inset) When capsaicin binds to a nerve cell in the tongue, calcium ions flood inside the nerve cell, which causes it to release brain chemicals that lead to the activation of other nerve cells and ultimately to the brain signal that tells the person “It’s hot!”
Pain receptors are proteins that have a certain shape that only fit specific molecules. Some receptors have the correct shape for capsaicin to fit into, like a lock and key. When a capsaicin molecule binds to one of these receptors, calcium ions (Ca2+) flood in. This flood of calcium ions triggers the release of neurotransmitters that send a message to the brain. Neurotransmitters are chemicals that are transmitted from one neuron to the next. The brain interprets this message as pain. Capsaicin also stimulates those receptors that perceive heat, known as thermoreceptors.
You can build up a tolerance to eating hot foods. The general consensus is that the pain receptors in the tongue and the mouth become desensitized over time if you have eaten a lot of hot food, allowing you to eat increasingly hotter foods.
But if you have not worked a tolerance for hot peppers, not only will your pain receptors trick your brain into thinking you are being burned, but your body may mount an inflammatory response, as well. This response can cause your throat to swell, making it difficult to breathe, and it can damage your intestinal tract, as well. Also, if you eat too many hot peppers at once, you will likely throw up, as your body will try to eliminate the perceived toxin.
Capsaicin’s Many Uses
Capsaicin is used as a pain reliever, and it can be applied to the skin as a patch or a cream. It has been used to treat the pain of arthritis, shingles, and sore muscles. When capsaicin is applied to your skin, a steady stream of neurotransmitters is sent to the brain, stimulating pain signals in the body. Once these neurotransmitters are depleted, you no longer experience pain. You are exchanging short-lived intense pain for constant, low-level pain that your body gets used to. Once the nerve cells become depleted of neurotransmitters, they lose their ability to sense pain. But after you remove the capsaicin from your skin, the pain may return, because the neurotransmitters build up again.
It is generally accepted that peppers contain capsaicin as a defense against predators. It seems to play a role against certain types of fungus that are partial to hot peppers.
Eating Chili Peppers to Cool Down?
Chili peppers are prevalent in hot climates, and it is especially popular in Mexico and India. Why would you want to eat hot peppers if it is already hot outside? Wouldn’t they make you hotter? When you eat hot peppers, you tend to sweat. Sweating is a cooling mechanism for the body. As sweat evaporates, energy is removed from the body. Evaporation is an endothermic phase change, because energy must be absorbed to overcome the forces of attraction between the molecules in the liquid phase that are present in sweat, allowing them to enter the vapor phase, so sweat can turn into a gas. So, it makes sense that eating hot peppers would be a more common practice in warmer regions of the world.
Chili peppers are actually good for you. They contain three times as much vitamin C as oranges, and they are also loaded with vitamins A and E, as well as folic acid and potassium. There is some evidence that chili peppers can help people lose weight by raising their metabolism. In particular, capsaicin increases the rate of thermogenesis, the process by which cells produce body heat.
So the next time you decide to go wild and try the 5-alarm chili, make sure you can handle the 1-alarm chili first. Just make sure you have a large glass of milk on hand. No matter how intense the pain of consuming chili peppers, just remember that your mouth is not really on fire.
Selected References
- Williams, C. Pepper Power. ChemMatters, April 1995, pp 10–13.
- Helmenstine, A. M. How to Make Hot Peppers Stop Burning. About.com Chemistry: chemistry.about.com/b/2013/03/08/how-to-make-hot-peppers-stop-burning.htm [accessed Oct 2013].
- Raloff, J. Understanding Why Hot Peppers Are Slimming. Science News, June 3, 2010:www.sciencenews.org/view/generic/id/59930/description/Understanding_why_hot_peppers_are_slimming.html [accessed Oct 2013].
Brian Rohrig teaches chemistry at Metro Early College High School in Columbus, Ohio. His most recent ChemMatters article, “Keeping Cool, Staying Warm: How Animals Survive Temperature Extremes,” appeared in the October 2013 issue.