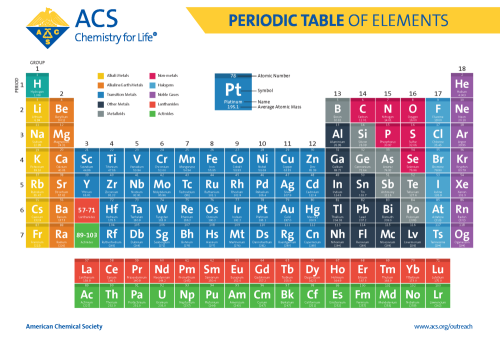
Periodic Table of Chemical Elements
The periodic table of chemical elements, often called the periodic table, organizes all discovered chemical elements in rows (called periods) and columns (called groups) according to increasing atomic number. Scientists use the periodic table to quickly refer to information about an element, like atomic mass and chemical symbol. The periodic table’s arrangement also allows scientists to discern trends in element properties, including electronegativity, ionization energy, and atomic radius.
Many scientists worked on the problem of organizing the elements, but Dmitri Mendeleev published his first version of the periodic table in 1869, and is most often credited as its inventor. Since then, the periodic table has evolved to reflect over 150 years of scientific development and understanding in chemistry and physics. Today, with 118 known elements, it is widely regarded as one of the most significant achievements in science.
Educational Resources
Browse activites and resources for students and educators from elementary school to post-doc, including a gallery of periodic tables.
SHOP
Use periodic table merchandise as prizes for creative uses of the periodic table, poem contests, art contests, and more.