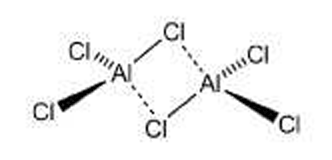
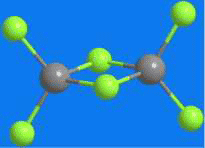
At first glance, aluminum chloride appears to be an inorganic salt with the empirical formula AlCl3; however, aluminum, like other group III elements, tends to form covalent bonds with halides. The energetically preferred coordination number of four causes AlCl3 to form the dimer Al2Cl6 shown, even in the vapor phase. AlCl3 is perhaps the quintessential Lewis acid; it reacts violently with water to form strongly acidic solutions. It has been used for decades in industry, most notably as a catalyst in Friedel–Crafts alkylation and acylation reactions, as well as in dozens of other processes. Its hexahydrate is the active ingredient in many antiperspirants.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

