Crest: A Breakthrough in Oral Care
Dedicated at Procter & Gamble in Cincinnati, Ohio, on April 3, 2024, and Indiana University in Bloomington on April 4, 2024
The history of toothpaste extends back to ancient times in Egypt. Concoctions to clean teeth included abrasive ingredients, such as charcoal and pumice, along with essential oils added to fight bad breath. Little changed over the ensuing centuries, and formulations in the mid-20th century were still little more than pleasant-smelling abrasives.
But then Crest toothpaste brought fluoride chemistry to the fight against cavities, launching a new era in dental care. Developed through a partnership between industry and academia, Crest improved the dental health of millions worldwide by putting powerful cavity-fighting chemistry in the hands of consumers.
Contents

Trial run
Trials of the new product were launched in the 1950s at Indiana University (IU), in Bloomington. There, a dentist instructed children to brush their teeth — in whichever way they normally did — with a new kind of paste. After six months, the kids came back for a checkup. These local children were not just dental patients, they were participants in one of the biggest clinical trials ever run on toothpaste. The main investigator in this endeavor was Dr. Joseph Muhler, a dentist and assistant professor in the university’s chemistry department. And it was his dream to take a bite out of one of the most pressing dental conditions in the U.S.: tooth decay.
At the nearby five-and-dime store, tubes of toothpaste lined the shelves. Madison Avenue spin doctors wrote advertising copy promising consumers fresh breath, a dazzling smile, and even protection against rotting teeth. Many oral health claims by the companies irked the American Dental Association because they overpromised the results that consumers could expect. The truth was that none of these products showed meaningful success in putting the brakes on cavities (“dental caries”), which were developing at a rate of hundreds of millions per year in the U.S.
In the late 1940s, when Muhler began his research — and for millennia before that — tooth decay was a major problem. As soon as a baby cuts its first tooth, millions of microbes in its mouth begin to feast on simple sugars and food particles, mixing with saliva and coating tooth enamel with plaque. As the bacteria multiply, they secrete lactic acid as a waste product.
Teeth are coated in enamel, the hardest material in the body — a crystalline form of calcium phosphate known as hydroxyapatite (Ca10(PO4)6(OH)2). The protective enamel layer is hard but not invincible. Acids made by microbes in the mouth eat slowly into teeth, eroding the enamel and then the softer dentin underneath. This can lead to pain, infections, abscesses, and sepsis. For thousands of years, a common fix was to pull a rotten tooth, which could be a very painful experience.

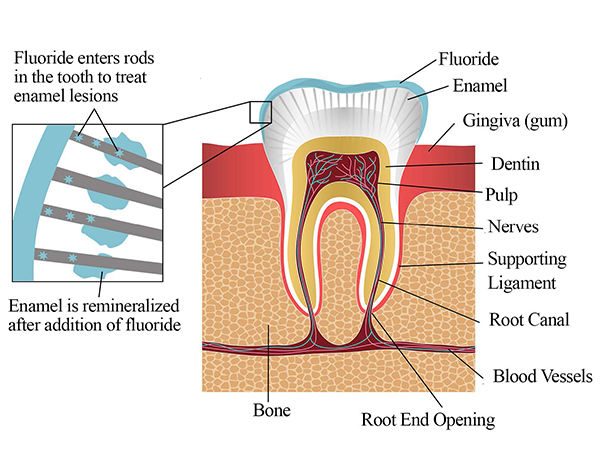
The reluctant dentist
As a child in Fort Wayne, Indiana, Muhler’s imagination was captured by chemistry. He took as many high school science courses as he could and dreamed of a future surrounded by test tubes and chemical reactions. Then World War II began, and the U.S. Navy had other plans for Muhler. He was almost finished with his undergraduate degree at IU when his draft notice arrived in 1944.
During both world wars, some men were rejected from military service because they couldn’t meet the minimum requirement of having six upper teeth matched by six in their lower jaw. The dental plague was so dire — tens of thousands were turned away over too few teeth — that the U.S. armed forces began seeking solutions. In 1912, on the eve of U.S. involvement in World War I, the U.S. Navy Dental Corps was launched with the intent to train front-line fighters in the war on tooth decay. Three decades later, this dental battle was still underway, and the Navy wanted Muhler to join the fight against cavities by offering him support to attend dental school.
He arrived at the Indiana School of Dentistry in Indianapolis in 1944, feeling ho-hum about a monotonous career filling cavities and pulling molars. “At the time I hadn’t the slightest interest in dentistry,” Muhler recalled.
Enter Harry Day, a chemistry professor at IU Bloomington, who was tasked with teaching biochemistry to first-year dental students like Muhler.
To capture their attention, Day later wrote, he emphasized topics related to dentistry. “The work on tooth decay and fluoride in the diet was a natural.”
For Muhler, the implication of chemistry impacting tooth decay was a game changer. Studying dentistry went immediately from dull to exhilarating. Fluoride and enamel were a match made in chemistry heaven.
At Bloomington, Day and Muhler gathered an assortment of chemicals and lab equipment for the biochemistry course. “Our stockroom was especially well supplied with various fluorides [as] a result of the work of another departmental chemist, F. [Frank] C. Mathers, who had hit upon a method of extracting the element while working for the U.S. Chemical Warfare Service in World War I,” Day wrote.
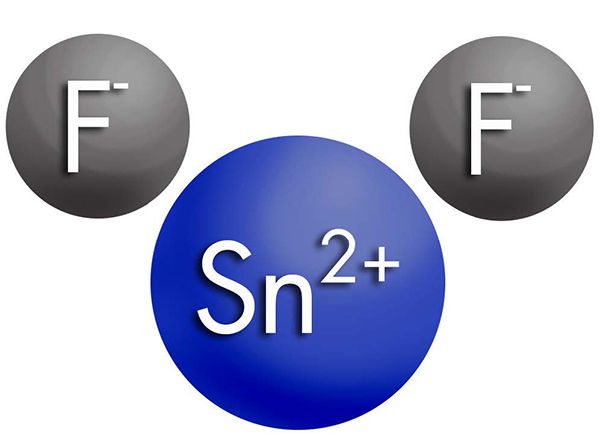
The pure form of fluorine, F2, is almost nonexistent in nature. Instead the element is typically found in the form of compounds with other elements or molecular groups. When combined with metals — such as tin or sodium — fluorine atoms take on a negative charge, while the metal atoms become positively charged. These negatively charged fluorine ions are referred to as fluorides.
Serendipity was central to the discovery that fluorides are effective against tooth decay.
The story goes back before Muhler was born. It was the early 1900s, and in the hills of Colorado, a bunch of kids in a young dentist’s office were fortunate enough to have almost no tooth decay. Dr. Frederick McKay noted his patients also had brown, stained teeth. He suspected the answer might be found in the local water. McKay sent a sample to Harry Churchill, a chief chemist at The Aluminum Company of America. Churchill found the water contained high levels of naturally occurring fluoride.
However, most natural water supplies don’t contain much fluoride. So in the 1930s, researchers led by Dr. H. Trendley Dean at the National Institute of Health looked into adding low levels of fluoride to drinking water and found no negative effects. And in 1945, the first test to fluoridate drinking water took place in Grand Rapids, Michigan.
The hope was that applying fluoride to developing teeth would create a more durable surface that would resist decay. And because fluoride reacts with tooth enamel, making it more resistant to acids and, therefore, less likely to decay and develop a cavity, the fluoridated water worked. Despite the evidence, there was resistance, and conspiracy theories arose about Communists trying to poison Americans en masse. To this day, these concerns over the hazards of ingesting fluoride in drinking water remain unproven, with organizations like the Centers for Disease Control and U.S. surgeon generals supporting this inexpensive way to fight tooth decay in communities.
Most bacteria in our bodies are harmless; some are even beneficial. Then there are the bacteria that cause oral diseases such as tooth decay, or cavities, and periodontal disease.”
— The True Story of Why You Get Cavities, According to a Billion Microbes; College of Dentistry, University of Illinois Chicago

The tin fluoride discovery
In 1948, Muhler received his dentistry degree. Still not sold on the idea of a dental practice, he decided to embark on a Ph.D. under Harry Day’s supervision. He began each morning by delivering newspapers to boost his small research salary. Then he worked from 6 to 7:30 a.m., grinding tooth samples and dousing them with various fluorides. After classes, he went back to the lab until midnight. Over the next two years, he meticulously tested more than 300 fluoride compounds, searching for the best combination of ions to preserve enamel. Out of all the pairings, he found a type of tin fluoride, known as stannous fluoride, that made enamel more resistant to acid than any other fluoride combination, including the most common substance then used by dentists: sodium fluoride.
Muhler was deeply religious and felt a calling to help underserved communities. In a 1954 interview, he said he regarded fluoridated water as “the best decay-prevention measure we know, the one way of making certain that all the kids in a town, rich and poor alike, are protected.” Yet he knew many households would never have fluoridated water, either because they used well water or their town didn’t add fluoride to the water supply.
Another option was for dentists to periodically apply sodium fluoride to children’s teeth. While this technique had positive results for protecting enamel, Muhler worried that few parents could afford the extra $15 charge on the dental bill. So the ultimate puzzle for Muhler, who was leading the charge toward preventive dentistry, was: How can we supply fluoride ions to those billions of teeth in the U.S. that don’t have access to fluoridated water?
Muhler’s next step was getting his tin-fluoride concoction into the mouths of mammals to test its effectiveness in a living system. Working with rats and hamsters, he showed that the treatment led to a big decrease in cavities in those species, compared to the control groups.
Procter & Gamble enters the picture
At the same time that Muhler and Day were working together to reduce cavities in lab animals, Procter & Gamble (P&G) product research head Verling Votaw was tasked with charting P&G’s future in oral hygiene products. Votaw had an interesting connection to fluoride: He had studied chemistry at IU under Mathers, who did the preliminary fluoride extraction work Muhler was leveraging. At P&G, Votaw’s main goal was getting a toothpaste with fluoride on drugstore shelves. That wasn’t yet possible, because the reactive fluoride ions in the company’s experimental pastes immediately bonded to the polishing agents in the formulations, instead of bonding to tooth enamel, as desired.
Muhler, although still working on his doctorate, was a prolific publisher and presenter who was also perplexed by the enigma of keeping fluoride active once it was mixed into toothpaste. At a dental convention in Chicago where he gave a talk on his stannous fluoride research, he got Votaw’s attention. Votaw and P&G had a modern vision when it came to product development. Of the company’s three most important brands — Ivory, Crisco, and Tide — none had been entirely created in-house, and the development team was engaged in looking outside the company for additional talent to help develop other products. P&G was happy to fund Muhler’s work. In return, the company would produce and distribute the final product — if an effective fluoride chemistry could ever be discovered. The deal would share the patent proceeds with Muhler, his team, and IU.
In 1949, now with funding to support his lab, Muhler went back to work with a new IU professor of chemistry named William Nebergall. More than 500 combinations of fluorides and polishing agents were created and tested, but disappointments mounted. The indiscriminate fluoride ions bonded with any paste the researchers created, rather than the desired target — enamel. Muhler tried and tried again. He published dozens of studies with colleagues.
Months went by and no good news came from the various fluoride toothpaste tests. Then one day in 1950, Nebergall heard an IU dental researcher mention that solid substances become less reactive when heated. Nebergall went back to the lab and put conventional polishing agent ingredients in an oven, then accidently left them cooking overnight. After the mixture was removed from the oven and cooled, the fluoride ions didn’t combine with the polishing agent but instead remained freer to react with tooth enamel once applied to the teeth. At long last, the team had a solution. (Nebergall’s discovery was awarded a patent in 1959.)


A massive study
Muhler and team now had a fluoride delivery system. In 1951, to test how well it worked, he enlisted an army of Bloomington boys and girls, later known as the “Crest kids,” with the help of the Division of Dental Health of the State of Indiana and local school officials. He brought on moms and dads and university students. The tireless Muhler received his Ph.D. in chemistry in 1952.
Over the next few years, one-third of the city of Bloomington took part in the P&G/IU study. It was a logistical puzzle made trickier by some parents wary of what was in the mystery tube of toothpaste. The details of the experiment were confidential, so there wasn’t much the team could tell them. But the researchers tried to convey to volunteers that they were each vital to the experiment, Muhler said. “That wasn’t too hard because it was true.”
The study was so massive that by 1955, P&G had supplied more than 100,000 tubes of toothpaste. More than 9,000 mouths were examined and over 60,000 X-rays taken. Statisticians at Ohio State University, overseen by professor and P&G researcher Arthur Radike, counted the cavities — or lack thereof — and crunched the data. In the end, the evidence showed that stannous fluoride, combined with compatible abrasives, was a wonder-compound that reduced the number of cavities by one-third compared with the control group.
Now P&G had a new toothpaste, one that used chemistry to reduce cavities and positively impact consumer’s lives. But adding fluoride to prevent disease created a therapeutic agent rather than just a cosmetic one. To move forward, P&G needed the okay from the U.S. Food & Drug Administration, and that came in 1955. The company named the new product Crest - with Fluoristan, and then set out to convince people of the benefits of fluoride in toothpaste. Crest promised more than a clean and dazzling smile. It promised healthy teeth too.
Sales crept steadily upward, but Votaw and P&G were not finished with their marketing plan. They enlisted the admired Muhler to ask the American Dental Association for a statement validating Crest’s effectiveness (the ADA does not “endorse” products).
Over a five-year period, the Indiana scientist conducted requisite clinical trials and collaborated with the ADA to review their studies. And on August 1, 1960, the ADA’s Council on Dental Therapeutics issued their evaluation of Crest toothpaste:
“After careful consideration of the results of clinical studies conducted on Crest toothpaste, manufactured by the Procter & Gamble Company, the Council on Dental Therapeutics has recognized the usefulness of the dentifrice as a caries preventative agent.”
The dental plain-speak may have sounded mundane, but the recognition from the ADA solidified Crest’s cavity fighting value in the minds of Americans. Soon, Crest was outselling every other brand. Money for licensing the patent to P&G flowed to the university and helped build the Oral Health Research Institute at IU’s School of Dentistry.
Muhler’s passion for chemistry didn’t end in the dental office. He fell in love with farming while at his Michigan vacation cottage and endeared himself to skeptical local farmers when he used chemistry to increase corn yields in the sandy soil by 300 percent. He painted his son’s teeth with all kinds of fluorides to test their tenacity. “I’m 72 and to this day, I have never had a cavity,” says Joseph Muhler II, a family physician in Fort Wayne, Indiana. He remembers his dad as the quintessential absent-minded professor who worked 16-hour days exploring chemistry to create big inventions. “My mother was saintly and just put up with his quirks,” he says. “She was infinitely patient and the perfect soul mate.”



A growing legacy
Crest has solidified billions of teeth against decay — and its place in history. Today P&G continues to improve its line of Crest products using both stannous fluoride and sodium fluoride to fight enamel erosion, cavities, gingivitis, plaque, sensitivity, tartar, staining, and bad breath — all the challenges bacteria-filled mouths and dietary habits can offer. These and other products with fluoride have become important tools in promoting dental health around the world, particularly because many countries and municipalities do not fluoridate water. And it all began with Crest.
Our findings have resulted in benefits to oral health for millions of people in many parts of the world.”
— Harry G. Day, The Story of the First Fluoride Toothpaste, Chemical Heritage, Fall 2001
Landmark dedication and acknowledgments
Landmark dedication
The American Chemical Society (ACS) designated Crest as a National Historic Chemical Landmark (NHCL) in a ceremony at Procter & Gamble in Cincinnati, Ohio, on April 3, 2024, and a ceremony at Indiana University Bloomington on April 4, 2024. The commemorative plaque reads:
Until the mid-20th century, toothpastes were little more than pleasant-smelling abrasives. But in 1955, the introduction of Crest toothpaste brought fluoride chemistry to the fight against tooth decay, launching a new era in dental care. Developed through a partnership between Indiana University and Procter & Gamble, Crest with stannous fluoride was the first toothpaste recognized by the American Dental Association for being effective against cavities. Since that time, fluoride toothpastes have improved the dental health of billions worldwide by putting powerful cavity-fighting chemistry in the hands of consumers. And that all began in the chemistry labs at Indiana University.
Acknowledgments
Written by Victoria Bruce.
The author wishes to thank contributors to and reviewers of this booklet, all of whom helped improve its content, especially members of the ACS NHCL Subcommittee.
The nomination for this Landmark designation was prepared by ACS’ Cincinnati Local Section, with sponsorship by Procter & Gamble and Indiana University.
Second Procter & Gamble Landmark
The National Historic Chemical Landmark for Crest is the second such designation at Procter & Gamble. ACS awarded the company’s first Landmark for the development of Tide, which was recognized in 2006 as the first heavy-duty synthetic detergent.


