Jellyfish have simple, gelatinous bodies made mostly of water, but they are harder to raise than you might think. Just ask Jazmine Sorenson, a recent high school graduate in upstate New York, who did just that over the course of her senior year. She took a unique independent studies class that gives students at Berlin High School a small taste of what marine science is like.
“I never thought I would have the opportunity to raise animals for an aquarium,” she says. “It was like a dream come true.”
Sorenson was able to fulfill that dream thanks to her school’s grant-funded program that teaches students the science behind growing jellyfish by actually doing it. The class also includes visits to aquariums across New England, including a few behind-the-scenes tours.
“On our first visit, I remember the variety of species, and being amazed by the animals’ colors and sizes,” she says.
Jellyfish in particular are among the most captivating creatures in aquariums. The animals’ translucent bodies pulse gracefully, resembling ghostly aliens. With no shells, brains, hearts, or teeth, the squishy creatures seem rather vulnerable to predators, and yet they are often the ones doing the predation.
Figuring out how to raise jellyfish in an aquarium gives students a better appreciation for ocean chemistry, how it sustains life, and a way of thinking about solutions to some of the problems facing marine ecosystems today.
Mimicking the ocean
Jellyfish have been around for at least 500 million years. They live in every ocean, and appear to be on a trajectory to survive for many more years. The marine animals are even flourishing while other sea life is threatened by climate change and overfishing.
Providing a tank of water for such simple and hardy creatures to inhabit might seem straightforward. But before Sorenson could receive her jellyfish to put in the tanks, she and her classmates discovered that maintaining an optimal aquarium environment requires a careful balance of salts, acidity, and minerals.
“Seawater is much more than pure water with salt; it’s a complex mixture of soluble minerals that interact,” says Sorenson’s science teacher, Matt Christian. “The students have to learn how to measure the levels of different elements in the water and understand how to make adjustments, so that the animals don’t die.”
On the first day of class, Sorenson and her peers split into groups of two or three. Each team was given 5 gallons of tap water in a bucket, a scale, a scoop, and a pail of sodium chloride (NaCl) to add to their aquarium. Sodium ions (Na+) and chloride ions (Cl-) account for more than 90% of the dissolved ions in seawater.
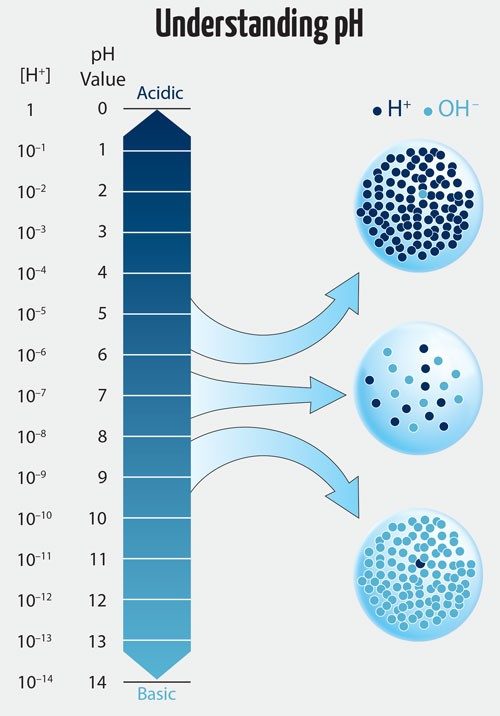
The pH scale runs from 0 –14, with 7 being neutral. The lower the pH, the more acidic the solution, and the greater the pH, the more basic. What the pH of a solution tells you is the relative amount of hydrogen ions (H+) and hydroxide ions (OH-) in the solution. A solution with more hydrogen ions is acidic; one with more hydroxide ions is basic.
Once they mixed the water and salt in their tanks, the testing began. They started with pH, the measure of how acidic or basic an aqueous solution is. The pH of the water needed to measure between 7.8 and 8.6, Christian says. Anything outside of that would jeopardize the health of the ecosystem.
When the pH was too low, they added limewater [Ca(OH)2(aq)], which is basic. If the pH was too high, the students added hydrochloric acid (HCl) or vinegar (CH3COOH).
“It was always nerve-racking because if you did one drop wrong, it could change the entire test result, and you would have to start over,” Sorenson says. “We were always rechecking what we were doing before coming to the conclusion that the level was fine.”
In addition to pH, the levels of calcium ions (Ca2+) are important. Calcium makes up 0.04% of seawater by mass, and is an integral ingredient in the shells produced by some sea life. To monitor calcium ion levels in their aquariums, students used titration. Titration is a common lab method that involves the slow addition of a solution with a known concentration to an unknown solution until there’s a color change.
A calcium titration begins by adding an indicator known as Eriochrome black T (EBT) to a sample of aquarium water. This indicator changes color from blue to red-violet when it combines with a metal ion such as calcium. Next, a standardized solution of ethylenediaminetetraacetic acid (EDTA) is slowly added to the sample of aquarium water. EDTA has a stronger affinity for the metal ion than EBT and displaces the indictor from the metal ion. As this happens, EBT changes color from violet to a pure blue color again, indicating the end of the reaction. What’s known in this reaction are the following: the concentration of EDTA solution, the volume of EDTA solution added, and the volume of the aquarium water sample. Through stoichiometry, the concentration of calcium ions in the aquarium water can be calculated.
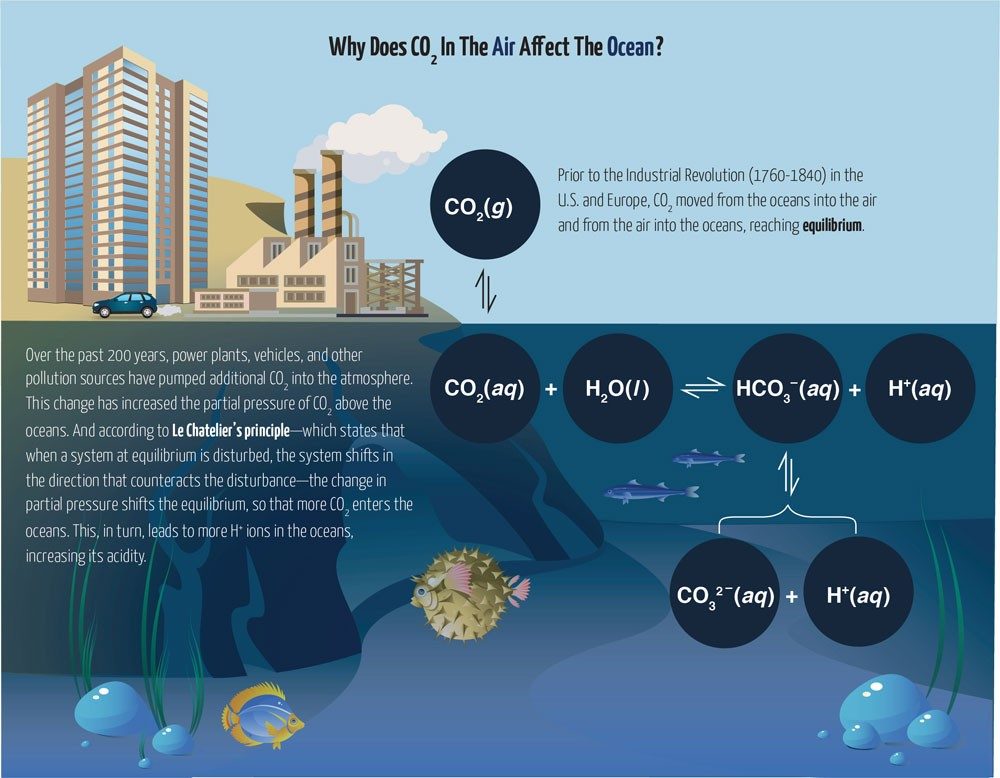
Students also needed to figure out how to maintain a stable pH in their aquariums. The level of acidity can increase as sea animals release waste. Christian’s class used sodium bicarbonate (NaHCO3) for this purpose.
Sodium bicarbonate dissociates into sodium (Na+) and bicarbonate (HCO3-) ions. When an acid is added, the bicarbonate ion will react with the H+ from the acid:
H+ + HCO3- ⟶ H2CO3
The H2CO3 forms carbon dioxide and water (see sidebar above), and the CO2 leaves the aquarium tank, resulting in a nearly constant pH. The bicarbonate ion will likewise resist a change in pH when a base is added by removing the OH- ions and forming water:
OH- + HCO3- ⟶ H2O + CO32-
Because of the calcium ions present in the seawater, excess carbonate ion is removed from solution as insoluble CaCO3(s), keeping its concentration low and the pH stable.
Ready for sea life
After several weeks of routine testing and adjusting of pH and mineral levels, Sorenson and her teammates finally received pinhead-size jellyfish polyps from the local aquariums.
“They looked like tiny dots of paint when we got them,” Sorenson says.
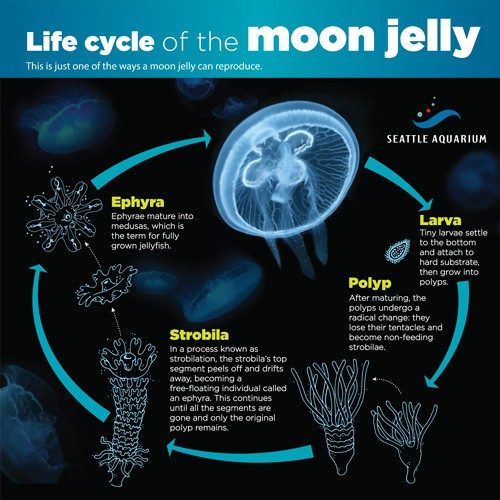
A jellyfish polyp isn’t what we normally picture when we think of jellyfish, freely swimming in the ocean. A polyp attaches to an underwater surface, and under ideal conditions, it elongates and reproduces asexually by budding off clones of itself.
Each polyp sheds between 10 and 15 immature jellyfish called ephyra that average two millimeters in diameter, about the thickness of a nickel. Under optimal conditions, the ephyra grow into adults, taking on the recognizable bell-shaped form jellyfish are known for. In three months, they reach teacup size and are ready to go back to the local aquarium. But it isn’t an easy task.
“We had difficulty raising them,” Sorenson says. “We had plenty grow, then shrink or die.”
Keeping proper water conditions wasn’t the only challenge. Some factors were out of the students’ hands. The school experienced a handful of weekend power outages that shut off the lights, bubblers, and pumps. Following the blackouts, students needed to check and adjust their water conditions again.
Beyond the aquarium
The point of the class, however, isn’t solely to learn how to take care of cool animals. The students get a glimpse into how concepts they learn in class might apply to real-life problems: For example, how might scientists approach the dropping pH in the world’s oceans? Over the past 200 years, ocean pH has decreased from a pH of 8.2 to 8.1. This sounds like a small difference, but because the pH scale is logarithmic, a drop of 0.1 pH units represents a change in hydrogen ion concentration from 6 x 10-9 M to 8 x 10-9 M.
Like the students in Christian’s class, some scientists have asked: What can be added to the ocean to keep its pH balanced?
Researchers have floated several ideas to reduce the ocean’s CO2—and its acidifying effect—including adding things to it, such as limestone (CaCO3), other minerals, or iron (“iron fertilization”). Limestone and other minerals dissolve and consume CO2. The idea behind iron fertilization—also called iron dumping—is that iron, an important nutrient to phytoplankton, would fuel a boom in the organisms’ growth. Phytoplankton, which are microscopic marine algae, consume CO2 through photosynthesis to produce glucose, removing CO2 from the water.
6 CO2 + 6 H2O + Sunlight ⟶ C6H12O6 + 6 O2
But there are concerns that adding tremendous amounts of minerals or iron to make a measurable difference on ocean CO2 concentration would require too much energy or have unwanted side effects.
A much lower-risk approach to lowering ocean CO2—at least along coastlines—involves planting seagrasses, which like phytoplankton use CO2 for photosynthesis.
Natural beds of some of these plants have been deteriorating over recent decades due to water pollution. Restoring them would not only serve to remove CO2 from local waters, but it would also bring back essential habitats for other sea life.

An ocean’s worth of knowledge
After almost eight months of work in class, Sorenson and her classmates delivered their jellyfish to the local aquarium. The nearly year-long project was filled with a range of emotions from excitement to disappointment, as they struggled to maintain stable levels of salt, pH, and minerals in their tanks.
“We never knew what to expect, but it was exciting seeing the jellyfish get as big as they did,” Sorenson says. “I was proud but also sad when we had to bring them to the aquarium because we put a lot of effort into them.”
Sorenson and the others in her class learned how even a simple saltwater aquarium system requires constant adjustments to maintain the right balance of chemicals to keep their handful of organisms alive. Imagine taking those lessons beyond the classroom to appreciate the incredible complexity of ocean chemistry with entire ecosystems at stake.
REFERENCES
Ocean Acidification. National Oceanic and Atmospheric Administration. https://www.noaa.gov/education/resource-collections/ocean-coasts/ocean-acidification [accessed Oct 2020].
Hale, G. Acidic Seas: How Carbon Dioxide Is Changing the Oceans.” ChemMatters, Feb/March 2018: https://www.acs.org/content/acs/en/education/resources/highschool/chemmatters/articles/acidic-seas-how-carbon-dioxide-is-changing-the-oceans.html [accessed Oct 2020].
Emerson, D. Biogenic Iron Dust: A Novel Approach to Ocean IronFertilization as a Means of Large-Scale Removal of Carbon Dioxide from the Atmosphere. Frontiers in Marine Science, Feb 7, 2019: https://www.frontiersin.org/articles/10.3389/fmars.2019.00022/full [accessed Oct 2020].