Do you remember being fascinated by magnets as a kid? Using a magnet from your fridge, you could pick up small metal objects such as paper clips, as if by magic. But other metal objects, including pennies, wouldn’t budge under a magnet’s pull.
One idea you might have tried is to draw a liquid to a magnet, which would have been even cooler to see. You might not have succeeded, but it is possible! And, surprisingly, such liquids can be found within familiar items, such as dollar bills, ball bearings, and increasingly, in artwork. These intriguing liquids are called ferrofluids.
Ferrofluids are always jet black and mesmerizing to look at. If you hold a powerful magnet close to a ferrofluid, it forms liquid spikes. The spikes reveal the otherwise invisible magnetic field lines surrounding the magnet.
So, what are these substances anyway? You might notice that the term ferrofluids is similar to the Latin word for iron, ferrum. This is where the symbol Fe comes from. While not pure iron, ferrofluids do contain iron, usually in the form of iron oxide (Fe3O4), which is usually called magnetite.
Magnetite is commonly found in nature. The easiest way to find it is to make a slurry of soil and water and then stir it with a powerful magnet. The bits of hard material that collect on the magnet are likely magnetite. Lodestone, a naturally occurring magnetic mineral, is a type of magnetite that was used in the world’s first-known compasses.

The Nature of Attraction
To understand why magnetite is attracted to a magnet, we have to first look at the electron configuration of iron.
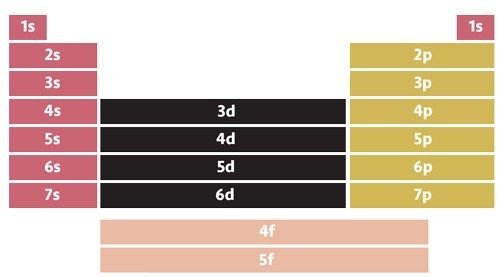
An electron configuration is the arrangement of electrons around the nucleus of an atom. Electrons occupy shells around a nucleus corresponding to specific energy levels. Shells closest to the nucleus are lower in energy than those that are farther from the nucleus.
Each shell is assigned a number and letter. The number, or coefficient, refers to the principal energy level. The letter (s, p, d, and f) refers to the subshell within the energy level.
A superscript refers to the number of electrons within a subshell. So, a configuration of 1s2 means there are two electrons in the s subshell of the first major energy level. Iron atoms have an electron configuration of 1s22s22p63s23p63d64s2.
In magnetite, Fe3O4, some of the electrons from the iron have been donated to oxygen to form oxide, O2-. To balance the eight electrons received by the four oxygens in Fe3O4, one of the iron atoms has given up two electrons, to form Fe2+ or Fe(II), while the other two iron atoms have each given up three electrons to form Fe3+ or Fe(III). Fe(II) loses its 4s electrons and has a configuration of 1s22s22p63s23p63d6, while Fe(III) loses an additional 3d electron and has a configuration of 1s22s22p63s23p63d5.
Each subshell is further divided into orbitals, which are regions of space in which electrons are most likely to be found. Any orbital can hold a maximum of two electrons. The d subshell contains five orbitals and can hold a maximum of 10 electrons.
Within an orbital, electrons create tiny magnetic fields as if they’re spinning. One electron creates a magnetic field oriented in one direction, and the other electron’s magnetic field is oriented in the opposite direction. The two opposite directions, called spins, are denoted by an upward or downward arrow.
According to Hund’s rule, each orbital within a subshell must be filled by one electron before a second electron can be added. Since electrons are negatively charged, they repel one another, so it is more energetically favorable for an electron to occupy its own space than for two electrons to crowd into a single orbital.
An orbital-filling diagram depicts the arrangement of electrons within their orbitals.
For example, the diagram below shows electrons’ orientations and distribution in iron’s d-subshell in an Fe(II) ion:
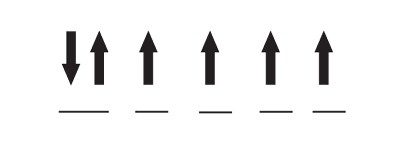
In the diagram, each horizontal line represents an orbital. With six electrons fitting into five orbitals, only one orbital is completely filled, while the others are partially filled. This arrangement influences a material’s magnetism because when two electrons are in the same orbital, they will have opposite spins that cancel out each other’s magnetic fields. The other unpaired electrons on an atom all line up in the same direction, so their magnetic fields reinforce each other.
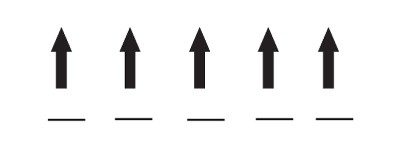
Iron(III) has one fewer electron than iron(II), so it actually has one more unpaired electron and thus a larger magnetic field:
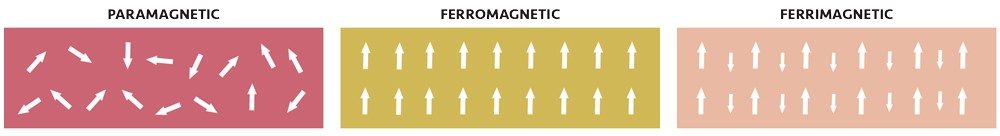
In most materials that contain iron(II) or iron(III), the atoms act like tiny magnets, but each atom’s magnetic field is oriented randomly relative to the nearby atoms. If you place it near a permanent magnet, there is only a slight tendency to line up the atoms’ fields relative to the external magnet, resulting in a slight attraction to the magnet (usually too small to notice if you hold the magnet to the material). Such a weak attraction to magnetic fields is called paramagnetism.
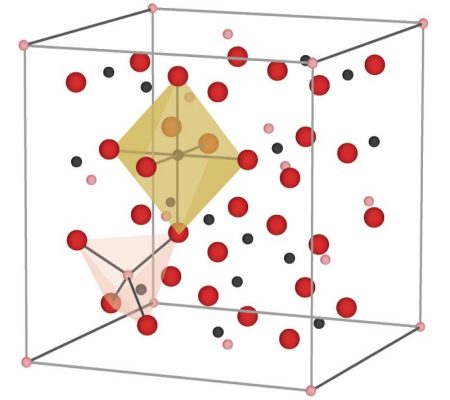
But in magnetite, there is something more going on. The spins of neighboring iron atoms in magnetite are not oriented randomly, but rather are determined by the directions of their neighbors’ spins. One-third of the iron atoms (which are Fe3+) in magnetite are each surrounded by four oxygen atoms, forming the shape of a tetrahedron. The other two-thirds of the iron atoms (the other Fe3+ and the Fe2+) are surrounded by six oxygen atoms each, forming the shape of an octahedron.
The tetrahedral iron atoms have their spins oriented strictly opposite to the octahedral iron atoms, forming a sort of three-dimensional checkerboard of spins going in alternating directions. But because the tetrahedral iron has only five spins while the octahedral irons have a total of nine spins, that means that for every three iron atoms, there is an excess of four spins pointing in the same direction. Multiply that by the trillions of atoms in even the smallest chunk of magnetite (such as the tiny particles in ferrofluids) and those spins add up to a macroscopic little bar magnet. This kind of global ordering of spins to produce a substance that is now strongly attracted to a magnetic field is called ferrimagnetism.
If all the spins are oriented the same way (rather than just an excess of one kind), it is called ferromagnetism, and it produces similar effects. Metallic iron, cobalt, and nickel are all ferromagnetic.
What about the oxygen atoms in magnetite? Neutral oxygen atoms have the electron configuration 1s22s22p4, so with three orbitals in each p subshell they have two unpaired electrons. But the oxide ions in magnetite have two added electrons and all their subshells are filled up (1s22s22p6). There are an equal number of electron spins in opposite directions, so the oxide ions do not contribute one way or another to the magnetism. If all the atoms in a substance are like this, the substance is weakly repelled by magnetic fields (usually too weakly to notice) and is said to be diamagnetic. Paper and plastic are both diamagnetic.
What are Ferrofluids?
Now that we have the magnetism part down, let’s take a closer look at what ferrofluids are. Ferrofluids are not true solutions, which are always transparent, nor are they merely temporary suspensions, which settle over time. Rather, they are colloidal suspensions—usually known simply as colloids—the particles of which remain permanently suspended.
Colloids always have two phases—the dispersed phase and the continuous phase. In ferrofluids, the magnetite particles are dispersed in an oil-based liquid as the continuous phase.
Colloids typically have particle sizes up to 100 nanometers (nm), one-thousandth the diameter of a human hair. In a ferrofluid, a typical magnetite particle will have a diameter of about 10 nm. These particles can be ground up from magnetite or chemically synthesized.
A typical ferrofluid is about 5% magnetite and 85% oil-based liquid by volume. Making up 10% of the mixture is a surfactant. The surfactant is a compound that coats the outside of the magnetite particles and helps keep them dispersed in the oil. This stops them from clumping into chunks that would settle out of solution. Oleic acid (a fatty acid, C18H34O2) and soy lecithin (a common food emulsifier, C35H66NO7P) are two common surfactants used in ferrofluids.

From Rocket Fuel to More Earthly Uses
Ferrofluids were originally developed by NASA scientists in the 1960s who were faced with a challenge: how to move rocket fuel from the tank to the combustion chamber in zero gravity. One engineer proposed infusing the fuel with magnetic particles, and then using a powerful magnet to draw the fuel into the combustion chamber. As ingenious as this idea was, it would ultimately be rejected. The addition of the magnetic particles reduced the fuel’s efficiency. In space exploration, every bit of weight is significant, and less-efficient fuel means you need more of it.
But one idea’s failure led to the successful use of ferrofluids in a wide range of other applications.
One of the most common uses that you would be able to test yourself is ferrofluids’ use in currency. If you hold a powerful magnet near the end of a dollar bill, it will be drawn to the magnet. This trick is possible because the ink used to print the bill contains a bit of ferrofluid. Magnetic ink is one of the many anti-counterfeiting features found in any denomination of U.S. currency—a non-magnetic bill inserted in a vending machine would be rejected.
Ferrofluids are also being tested for use in other areas. For example, they could help clean up oil spills. Ferrofluids are oil-based and readily stick to petroleum (“like attracts like”), which can then be extracted from the water using powerful magnets.
Ferrofluids have also shown great potential in the medical field. One promising type of cancer therapy called magnetic nanoparticle hyperthermia involves injecting ferrofluid into a cancerous tumor and then subjecting it to high-powered, alternating magnetic fields. This heats up the magnetic particles in the ferrofluid, and that energy is passed on to the cancer cells and kills them.
Their versatility, along with their unique liquid spikes, makes it hard to resist the magnetic pull of ferrofluids. A tube of ferrofluid and a magnet are not only entertaining to play with, but they could also possibly save a life one day.
From Failed Rocket Fuel to Everyday Uses and Beyond
Ferrofluids were invented for NASA’s space program in the 1960s, but they failed as a rocket-fuel additive, the application they were initially intended for. But the ingenuity of the material spurred engineers and scientists to find a variety of other uses for it.

CURRENCY
As an anti-counterfeiting measure, U.S. dollars are printed with ink containing small amounts of ferrofluids.

LOUDSPEAKERS
Another common use of ferrofluids is in loudspeakers. The magnetic material draws heat away from voice coils and dampens unwanted vibrations for a clearer sound.

COMPUTER CHIPS
Ferrofluids have been used in the production of semi-conductor chips for electronics. Manufacturers use the material to create a seal around components to keep air, dust, and water vapor out.
MEDICAL TREATMENTS
Researchers are investigating whether they can take advantage of ferrofluids’ magnetic properties to target the delivery of medicine to specific sites in the body—such as directing anti-cancer drugs to a tumor.
REFERENCES
Berger, P. Preparation and Properties of an Aqueous Ferrofluid. Journal of Chemical Education; 1999, 76 (7), pp 943–948.
Livingston, J. D. Driving Force: The Natural Magic of Magnets; Harvard University Press: Cambridge, Mass., 1996.