Take a minute to think about what you’re wearing right now. Not the colors or cuts of fabric you grabbed out of your closet this morning—but the textiles your clothes are made of.
Before your clothes became clothes, they were raw resources that were collected, processed, woven into textiles, then cut and sewn into the garments on your back. And their life cycle doesn’t end there. Nearly 90% of clothing takes an inevitable trip from closet to landfill. The problem is that although this process provides short-term convenience for customers and the fashion industry, in the long run, it’s not sustainable. Making and transporting clothes consumes raw materials and, at every step in the process, emits greenhouse gases.
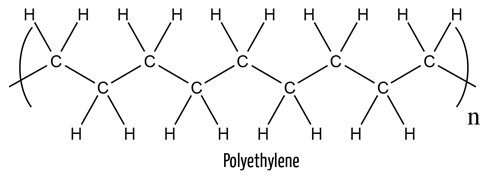
Does it have to be that way? Or can we reduce the fashion industry’s environmental footprint by recycling used clothes, like we do with plastic bottles? Plastic bottles consist of polymers such as polyethylene, (C2H4)n, a derivative of fossil fuels.
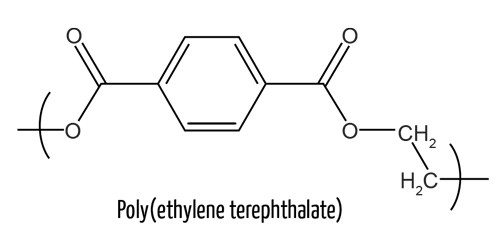
Your clothes are not much different. Many synthetic materials, such as polyester, are also made of polymers derived from fossil fuels, which are a nonrenewable resource.
But while it’s common for plastic to be collected for recycling, clothing recycling is a rarity. Recognizing that textile manufacturing and waste is a growing problem, however, researchers and some in the fashion industry are working hard to fix this.
“In order to keep materials in use for everybody, we’ve got to be able to reuse them and remake them,” says Kate Goldsworthy, co-director of the Centre for Circular Design at University of the Arts London.
The question is, what will it take to meet this goal?
Clothing chemistry
For Finnish chemical engineer Simone Haslinger, pursuing textile-recycling research was an opportunity to help tackle serious environmental problems.
“I really saw that [clothing recycling] can be useful, and that this is something that can help everyone,” Haslinger says. “It’s so important because we’ve had all of these climate change issues.”
The textile industry churns out the equivalent of 1.2 billion metric tons of planet-warming carbon dioxide (CO2) every year, according to a 2017 analysis from the Ellen MacArthur Foundation. That’s 30% more than all the world’s commercial flights. The industry also guzzles about 93 billion metric tons of clean water annually—roughly half the amount that the U.S. population drinks. Additionally, the world is on track to triple clothing production by 2050. Because 97% of source materials in clothing production are new, not recycled, the growing demand places enormous pressure on land, energy resources, and water.
Heaping our unwanted clothing in landfills also creates an environmental burden. As natural fibers degrade, they release methane (CH4), a potent greenhouse gas. Disposing and washing fabrics also releases microfibers, dyes, and specialized coating chemicals into the environment.
To help curb the environmental burden of fashion, Haslinger worked as a Ph.D. student at Aalto University in Finland on developing techniques to recycle old clothes. She now designs sustainable kids’ wear as a scientist for the Reima label.
Haslinger says that many technologies exist to break down and rebuild specific polymers, but the biggest lingering challenge is clear: “It’s the multitude of different materials.”
While the fibers in clothes are all polymers, long chains of chemically linked molecules, called monomers, there are many different types. Polyester fibers, for example, are in durable fibers found in 55% percent of textiles. Polyester is inexpensive, but producing it requires a lot of energy.
Cotton fabrics, including denim, make up 27% of all textiles and consist of biological polymers called cellulose. In nature, cellulose strengthens plants’ structures and is the most abundant polymer on Earth.
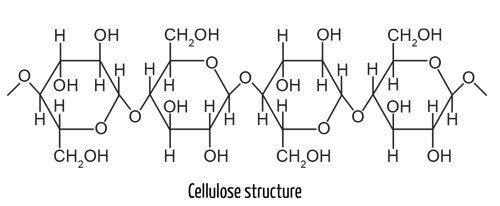
Most of the cellulose we use in clothing is manufactured from cotton plants, which require a lot of processing. Approximately 20,000 liters (5,000 gallons) of water go into making a pair of jeans.
Another 5% of textiles contain human-made cellulosic fibers. Lyocell is a popular example of this type of material these days. It can be produced from wood scraps or eucalyptus trees that require far less water and pesticides than cotton plants. Producing lyocell, however, isn’t completely harmless, as it is very energy intensive.
The bottom line is that resources are limited, and using them has a cost to the environment, while demand is increasing for these finite resources.
“The stuff on Earth is all we’ve got,” Goldsworthy says. “The population is growing, and of course, even that will peak at some point. It’s just not possible to imagine that [making new clothes] can go on and on and on forever the way we’re doing it.”
After pumping so much energy and resources into textiles around the world, only 13% of it gets recycled. The majority cascades down the quality chain to live a second life as insulation or stuffing. Less than 1% of clothing loops back to be made into clothing again.
Part of the reason why clothes-to-clothes recycling is so rare is because companies can’t just take used clothes, shred them, and spin the shredded bits into strong new yarn. Polymer length equates to fiber strength, and the length shortens with use.
“When you spin it, [the length] reduces; when the consumer wears it and washes it, it reduces,” says Haslinger. By the time clothes get tossed out, their polymers are too short to turn back into a quality fabric.

New technologies
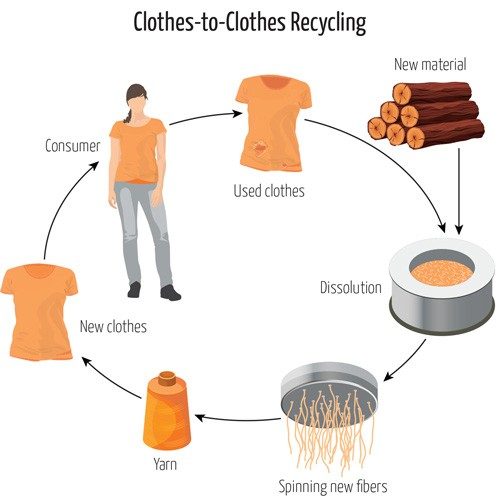
Enter solvent-based recycling. Although polymer fibers from old clothes are too short and weak from use to spin into new yarn through a mechanical process, there’s a way to reclaim the fibers for reuse. The underlying idea is simple: Collect used textiles, dissolve the polymers using solvents, and then spin the fibers into yarn.
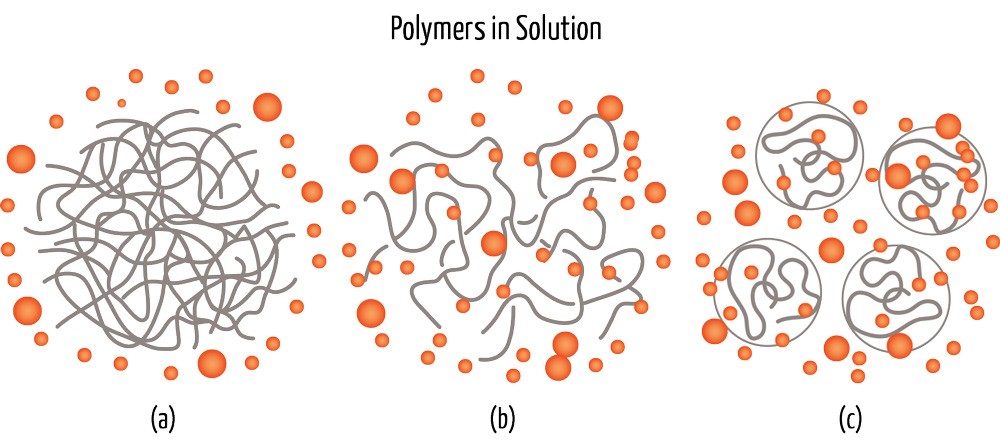
Unlike simple solutes such as sugar or table salt, polymers are chains of molecules that easily tangle, and they require special solvents to isolate them from each other. Imagine trying to dissolve a piece of denim in water—a fabric that dissolves in the washing machine isn’t going to be very useful! Even if you shred your pair of jeans into tiny pieces, intermolecular forces between polymer strands keep them together.
Finding the right solvent means making sure the polymer-solvent intermolecular attraction outweighs the polymer-polymer forces—the solvent should tug on the polymer molecules enough to detach strands from each other. In these processes, the groups of polymers untangle into individual strands and disperse within the solution. The result is usually a tinted clear liquid with a viscosity dependent on the size and concentration of the polymer molecules.
Haslinger’s doctoral work involved a technology called Ioncell, that dissolves cellulose from cut-up pieces of cotton clothes, wood, cardboard, or paper with 1,5-diazabicyclo[4.3.0]non-5-ene acetate—an ionic liquid.
To understand ionic salts, first imagine if table salt, NaCl, which normally melts at 801 °C (1,474 °F), could be liquid at room temperature. Its electrically charged cations and anions would exist as a fluid. Ionic liquids are somewhat similar to that. They are conductive salts with melting points below 100 °C (212 °F), but they are composed of an organic cation and either an organic or inorganic anion.
The cations and anions in ionic liquids like Ioncell’s readily engage in hydrogen bond interactions with cellulose. These attractive forces dissolve cellulose, converting it into a liquid form that’s easier to purify.
Liquid from the Ioncell process gets squeezed through tiny holes at high pressure, dripping threads of cellulose into a water bath. Unlike cellulose, the ionic liquid is soluble in water. So, when the polymer drips into the water, the solvent (the ionic liquid) washes away and the solute (cellulose polymer) goes back to sticking to itself to form solid fibers. The final steps involve stretching the fibers even thinner to make them suitable for clothes.
Ioncell shows that recycling cotton is possible. But the problem solving doesn’t end there. An additional challenge is that chemical recycling that works for cotton won’t necessarily work for rayon, nor is it guaranteed to break down cotton clothing at different stages, whether it’s new or been worn and washed.
Recycling processes are also sensitive to impurities. Additives such as spandex, water-repellent coatings, and dyes add to the chemical complexity. So, processing technologies often have to be optimized for a combination of factors, including impurity content, fabric quality, and the type of polymer.
Another approach to clothing recycling involves restoring a polymer to its original quality. This requires depolymerization. As its name suggests, depolymerization is a process that breaks down a polymer to its constituent monomers. This lets you rebuild it without waste, so that it’s as good as new.
The challenge ahead
Another challenge to recycling used clothing is that many of our clothes contain blends of different fibers.
“The more components you have—especially elastane—the harder recycling gets,” says Haslinger. Elastane, more commonly known as spandex, impairs recycling processes.
“That’s why I think all those that are now trying to commercialize [recycling are] saying: ‘We only focus on bedsheets,’ for example, or ‘We only focus on denim.’ Because they know [a broader approach is] very difficult,” Haslinger says. “None of them can say, ‘We’ll just take all of it,’ and recycle everything.”
And slowly but surely, through steady determination and innovation, scientists and clothing manufacturers are introducing recycled clothing fibers to the market. Adidas and Levi Strauss, for example, have released limited-edition garments containing NuCycl, a novel fiber made from 100% post-consumer cotton by U.S.-based company Evrnu. Finnish company Infinited Fiber teamed up with H&M Group to release a line of jeans in Europe that combines its regenerated textile fiber with organic cotton.
The vision for those developing all of these technologies is ultimately to build a circular economy, using waste as a resource rather than depleting raw resources to make new clothes and more waste. Understanding the chemistry of fabrics plays a huge role in closing that loop!
5 Ways to Reduce Your Fashion Footprint
What can we all do to make fashion more sustainable? Haslinger recommends being more conscious about the clothes you buy and avoiding those that you know will end up in the trash soon. “Try to keep your various [clothes] longer, maybe repair it, or buy from secondhand,” she says. “And if you buy something, try to buy something that is not blended that much.”
» Reduce demand by buying only what you need.
» Buy used clothes from consignment stores, thrift stores, or resale websites.
» Buy clothes that are built to last, and actually wear them—a lot! (How many items in your closet have you worn only a couple of times?)
» Avoid blended materials that current recycling technologies cannot handle (for example, spandex blends).
» Buy clothes from sustainable manufacturers that use recycled textiles and renewable
energy.
REFERENCES
Haslinger, S. et al. Upcycling of Cotton Polyester Blended Textile Waste to New Man-Made Cellulose Fibers. Waste Management, Sept 2019: https://www.sciencedirect.com/science/article/pii/S0956053X19305100?via%3Dihub [accessed Feb 2021].
Ellen MacArthur Foundation. A New Textiles Economy: Redesigning Fashion’s Future, Nov 28, 2017: https://www.ellenmacarthurfoundation.org/publications [accessed Feb 2021].
Water Sense: Statistics and Facts. U.S. Environmental Protection Agency, last updated Nov 7, 2018: https://www.epa.gov/watersense/statistics-and-facts [accessed Feb 2021].