Tanking up with Cooking Oil
By Beth Nolte
ChemMatters, April 2011
- Download article
- Teacher’s Guide
Word | PDF
Grease sloshes up the sides of the 5-gallon bucket I am carrying, and I dodge the splatter. I said I wouldn’t bring this stuff into my apartment. But here I am hauling a big bucket full of used vegetable oil back down three flights of narrow and twisting stairs!
I collect the oil free from my favorite Indian restaurant every week. Before I can use it in my car as fuel, all the floating chunks of food have to be filtered out. That’s the part I do in my apartment. I pour the oil into a filter bag suspended from a tripod over the 5-gallon bucket. Everything that flows through the filter can be used to run the car.
On the street outside my apartment, I open the trunk of my 1985 Mercedes Benz. Then all I do is unscrew the fuel cap, prop the funnel in place, and pour. Recycled oil becomes free fuel. Even though it’s a lot of work, I still love my vegetable oil car. Yet, I have to ask, is this worth the effort?
My boyfriend and I started this experiment because we wanted to try something different. After our car was stolen, we had the opportunity to rethink transportation. For months, we walked and biked, asked for rides, and took the bus. We contemplated cruising around town on a scooter. I even did my errands with a taxi cab! But let’s face it: Sometimes, having a car just makes life a lot easier.
We could not afford a new car, but I heard about diesel engines converted to run on straight vegetable oil. The idea of using filtered vegetable oil was something we could do ourselves and could afford.
We bought our car on eBay for $3,500. A friend of mine knew a person who could convert diesel engines to run on vegetable oil. So, we called him up, and for $1,500 more, he created the dual-tank system that we now have. The second tank used for vegetable oil was custom-made to fit into the trunk where the spare tire used to be.
Gasoline, diesel, vegetable oil: What’s the difference?
Gasoline, diesel, and vegetable oil have different compositions. Gasoline and diesel are each a mixture of hydrocarbons—molecules composed of only hydrogen and carbon atoms. But gasoline contains hydrocarbon molecules with 5 - 12 carbons while diesel contains longer molecules with 10 - 24 carbons. Examples of molecules present in gasoline and diesel are shown in Fig. 1 and Fig. 2, respectively.
Figure 1.
Isooctane is a hydrocarbon molecule often found in gasoline.
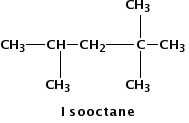
Figure 2.
Cetane is a typical hydrocarbon molecule found in diesel.
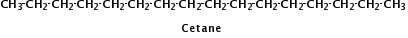
In both gasoline and diesel, the hydrocarbon molecules are attracted to each other through weak forces called intermolecular forces. But because the molecules in diesel are longer, they tend to bind more strongly than the smaller molecules in gasoline. As a result, diesel is more viscous than gasoline, that is, it is more resistant to flow than gasoline. Especially in winter, diesel has a thick consistency similar to the consistency of molasses or honey.
Vegetable oil, by contrast, contains fat molecules, which are composed of triesters. An ester is an organic compound formed when an acid and an alcohol react, releasing water. A triester is an organic compound produced in the chemical reaction of a molecule that contains three hydroxyl groups (–OH) called glycerol and three fatty acid molecules, each composed of long hydrocarbon chains (R) and a carboxylic acid group (–COOH):
Figure 3.
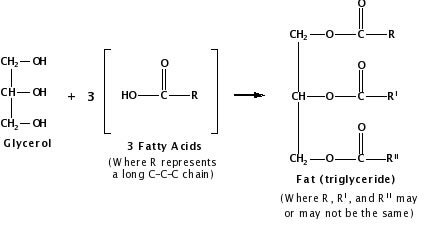
Depending on their structure and composition, fats may either be solid or liquid at room temperature. Although the words “oils,” “fats,” and “lipids” are all used to refer to fats, “oils” usually refer to fats that are liquids at normal room temperature, while “fats” refer to fats that are solids at normal room temperature.
The long hydrocarbon chains (R) in the vegetable oil molecules contain a large number of carbon atoms—typically 16 -18 atoms—so vegetable oil is closer in composition to diesel than to gasoline and is even more viscous than diesel.
Vegetable Oil Car
How does a car run on vegetable oil? Actually, it works like a car that uses gasoline or diesel. In all cases, the fuel—whether gasoline, diesel, or vegetable oil—is burned in an internal-combustion engine by reacting with oxygen from the air. In this process, the chemical energy available in the fuel is converted into mechanical energy, which moves pistons up and down inside cylinders. Each piston is connected to a crankshaft (Fig. 4 and Fig. 5) that creates the rotary motion needed to turn the wheels of a car forward.
In both a gasoline and a diesel engine, the fuel reacts with oxygen, producing gases and heat. The heat causes the gases to expand and explode. The major difference between the two types of engines is how this explosion occurs.
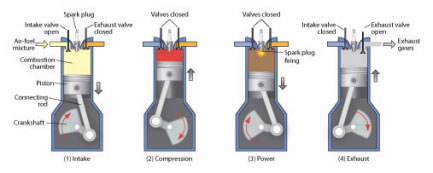
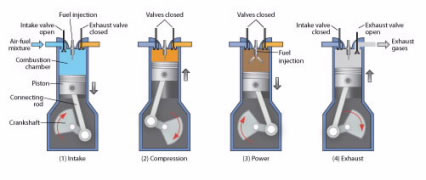
In a gasoline engine, a mixture of gasoline and air is first compressed by a piston (Fig. 4), and then a spark plug fires, igniting the mixture and causing it to explode. This explosion exerts pressure on the piston, pushing it downward and causing the wheels of the car to move. When the piston goes up, the exhaust products are released through a valve.
Figure 4.
A gasoline internal combustion engine works in four steps: (1) Intake: a mixture of air and gasoline is drawn in; (2) Compression: the mixture is compressed; (3) Power: a spark plug ignites the compressed gas, causing an explosion that forces the piston down; (4) Exhaust: when the piston goes up, it pushes out burned gases.
In a diesel engine, the air and the diesel are not mixed in the beginning. Air is drawn in first and is heated up by compression. Then, the diesel fuel is drawn in and the heat from the compressed air ignites it (Fig. 5), which causes the air/diesel mixture to explode. This explosion pushes the piston down, moving the wheels of the car. Then, the exhaust gases are released through a valve.
Figure 5.
A diesel internal combustion engine works in four steps: (1) Intake: air is drawn in; (2) Compression: the air is compressed, which heats it up; (3) Power: the diesel is drawn in, and the air diesel mixture explodes due to the heat from the compressed air; (4) Exhaust: when the piston goes up, it pushes out burned gases.
Why do these engines work so differently? One of the main reasons is that gasoline and diesel have different chemical compositions. When gasoline is heated, its relatively small hydrocarbon molecules separate from one another and enter the vapor phase. This allows them to react with oxygen molecules in the presence of a spark, releasing energy. This energy causes the gases produced in these chemical reactions to rapidly expand, creating an explosion.
Conversely, when diesel is heated, its long and tangled molecules do not separate as readily. It takes a larger amount of heat than with gasoline to separate them. In a diesel engine, this increased amount of heat comes from compressed air. This heat is needed to separate diesel molecules so they can react explosively with oxygen from the air.
Another important difference is that in a gasoline engine, gasoline burns in the presence of a spark while in a diesel engine, diesel burns without a spark. In a diesel engine, the source of heat comes from air under high pressure, which generates enough heat to ignite the vaporized form of diesel. The reason for this difference is that diesel engines were originally developed to be more efficient than gasoline engines.
How about vegetable oil? Because it is composed of long hydrocarbon molecules that are similar to those found in diesel, vegetable oil can only work in a diesel engine. As in the case of diesel, heat from compressed air causes the molecules in vegetable oil to react explosively with the oxygen molecules present in the air. A summary of the properties of gasoline, diesel, and vegetable oil is provided in Table 1.
Table 1. Comparison of the physical and chemical properties of gasoline, diesel and vegetable oil.
| Fuel Source | Gasoline | Diesel | Vegetable Oil |
| Chemical Composition | Hydrocarbon Molecules of 5-10 carbon atoms | Hydrocarbon Molecules of 10-20 carbon atoms | Fat molecules with hydrocarbon chains of more than 10 carbon atoms each |
| Viscosity | Low | High | High |
| Type of Car | Gasoline | Diesel | Diesel |
Why Convert Our Car?
We needed to convert the engine in our car because vegetable oil is more viscous than diesel. So, we needed to heat vegetable oil before it enters the engine. Heating vegetable oil decreases its viscosity. As the temperature increases, the vegetable oil molecules separate from one another, and the vegetable oil flows more readily.
In the fuel tank, which is located in the trunk of our car, the vegetable oil is heated with coils. The vegetable oil is carried from the tank to the engine through a hose that is also heated. This is done by surrounding the hose for the vegetable oil with a larger hose that contains a heating liquid. So the heat from this liquid keeps the oil warm until it reaches the engine.
When the engine is cold, I start the car on diesel. Once everything heats up, I flip a switch, and the hot grease starts flowing. The car runs best when the engine temperature is at least 80 °F.
Before I turn off the engine—especially if it is cold outside—I switch back to diesel to flush the vegetable oil out of the engine. Otherwise, the oil will cool down and harden in the engine. As you can imagine, if vegetable oil thickens in the engine, it would be hard to get the car started again!
Is Our Car Really Green?
We decided to use vegetable oil instead of diesel or gasoline because we wanted a car that was better for the environment. So how “green” is vegetable oil?
One clear advantage is that vegetable oil is “carbon-neutral,” meaning that the amount of carbon dioxide released when the vegetable oil burns is the same as the amount of carbon dioxide taken in by plants to grow. So these two effects—the burning of vegetable oil and the use of carbon dioxide by plants to grow—cancel each other out, resulting in no net increase of carbon dioxide in the atmosphere.
Another advantage of a vegetable car is that it pollutes less. For instance, compared to a traditional car powered by gasoline or diesel, our car produces 50% less carbon monoxide, 50% less hydrocarbons, and no sulfur oxides.
There are other sources of energy derived from plants with similar potential. For instance, scientists are exploring how to use a plant fat produced by algae called triacylglycerol as a fuel.
Scientists are also studying how to produce energy from cellulose, the fibrous or woody material of plants and trees made of carbon, hydrogen, and oxygen atoms. By removing oxygen atoms from cellulose, it would be possible to produce molecules that contain only carbon and hydrogen atoms. These molecules form what is known as “green” gasoline, a substance that packs more energy than cellulose.
Vegetable oil, algae oil, and green gasoline, generically known as biofuels, might change the way cars will run in the future. In the meantime, you can still use cooking oil!
Selected references
Pidwirny, M. Carbon Cycle. The Encyclopedia of Earth, Sept 16, 2009: http://www.eoearth.org/article/Carbon_cycle [accessed Dec 2010]
Wacker, T. Would You Use Veggie Oil to Fuel Your Vehicle? Mother Earth News, Dec 2007/Jan 2008, pp 111–115: http://www.motherearthnews.com/Green-Transportation/2007-12-01/Veggie-Oil-Cars.aspx [accessed Dec 2010]
Also in this Issue (April 2011)

A Single Ignition: A Cautionary Tale
Oil storage tanks have caused unexpected explosions and claimed the lives of many teenagers. How did these explosions happen?

Nuclear Energy: When Will the Lights Go Out?
Will nuclear energy keep us out of the dark until we develop new technology to provide enough energy to meet our needs? Find out.
Subscribe to receive full issue!
25 years of ChemMatters!

- Great for class projects, research, and science fairs
- Easy access to articles and puzzles from 1983-2008
Past Issues, Videos, Teacher’s Guides
Browse articles by topic
- Acids/Bases
- Atomic Theory
- Biography/History
- Bonding
- Equilibrium
- Food Chemistry
- Metals/Nonmetals
- Nuclear
- Organic/Biochemistry
- Periodicity
- Reactions
- Solids/Liquids/Gases
- Solutions
- Sustainability
- Thermochemistry

