Aluminum Recycling
By Tom Husband
ChemMatters April 2012
- Download article
- Teacher’s Guide
Word | PDF
In the Western world, recycling often feels like a chore. It is something we should do, like exercising and eating a healthy diet. This is in stark contrast to the situation in India where recycling is a livelihood for thousands of people who would otherwise have no way of making a living. The government does not run any recycling programs, and garbage is dumped in big containers outside of the cities.
Unemployed Indians often roam around the containers, looking for any material that can be recycled. The gathered waste is sold to small recycling plants that operate with basic technology and in poor conditions. Many of these plants are located in a slum town, called Dharavi, in Mumbai, the economic capital of India. Employees work long hours for low salaries, but they are grateful to have jobs in a country where unemployment is high.
Muktar Hamed works for India’s informal recycling sector. He is 15 years old and earns a living by recycling aluminum scrap. Every day, he wakes up just before 7 a.m., washes, eats breakfast, and then starts his work. He has an hour for lunch and then continues working until 8 p.m. His job is to melt the aluminum scrap in a furnace all day long. At the end of the day, Muktar sleeps in a bed that is right next to the furnace.
Hamed is one of thousands of Indians who live in Dharavi, one of the largest slum towns in South Asia, and make money by recycling and reusing nearly every aluminum material they can find. Not only do these people support themselves, but they also created a multimillion-dollar industry that is changing the regional economy and is allowing people to earn money.
Aluminum recycling in Dharavi
In Dharavi, aluminum recycling involves three important steps. First, aluminum products that have been used and thrown away, especially old soda and beer cans, are collected. Second, the cans are soaked in acid, so the designs and brand names are removed. Third, the cans are crushed and melted in a furnace.
Hamed works in a plant that receives crushed cans and then melts them in a coal-powered furnace. Once the cans have completely melted, the molten mixture is poured into molds, where it cools into solid aluminum bars called ingots. These bars are then transferred to another factory in Dharavi. There, they are melted again, and a special machine turns the liquid metal into blocks that are later used to make new aluminum items.
Hamed’s furnace is actually a hole in the ground, which is approximately one meter (3.3 feet) deep and half a meter (1.6 feet) wide. At the bottom of the hole is a vent, which is joined to the ground by a pipe. The purpose of the vent and the pipe is to enable oxygen to reach the lowest part of the furnace.
The furnace is filled with coal which is ignited and stoked to produce temperatures exceeding the 660 oC melting point of aluminum. Hamed places a crucible filled with the crushed scrap aluminum on the top of the furnace. The crucible is made of silicon carbide, which remains solid up to 2,730oC.
Why recycle aluminum?
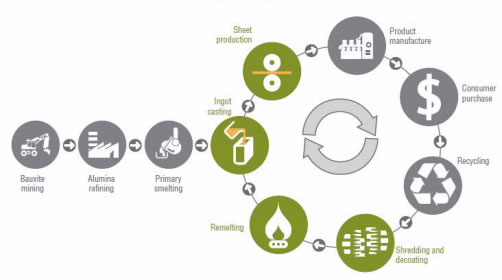
Aluminum recycling is profitable because extracting this metal from aluminum ore is expensive, it pollutes the environment, and it consumes a significant amount of energy. To understand how aluminum is used to make everyday products, let’s examine how an aluminum can is made (Fig. 1). When we recycle an aluminum can, we eliminate the initial steps, and the recycled aluminum becomes part of a cycle that can occur over and over again without loss of the aluminum’s properties.
Aluminum is extracted from an ore known as bauxite, which consists of aluminum oxide (Al2O3) and other compounds that contain aluminum, silicon, titanium, and iron. Aluminum oxide is separated from the other elements using the Bayer process, which consists of three stages. First, bauxite is dissolved in a solution of sodium hydroxide at high pressure and temperature. The resulting mixture contains a solution of sodium aluminate [NaAl(OH)4] and undissolved bauxite residues containing iron, silicon, and titanium.
Sodium aluminate forms through the chemical reaction of aluminum oxide with sodium hydroxide and water, as follows:

The residues sink gradually to the bottom of the tank and are later removed.
In the second stage, the sodium aluminate solution is pumped into a huge tank and cooled, and, as it cools, the sodium aluminate decomposes to form aluminum hydroxide [Al(OH)3] and sodium hydroxide:

The aluminum hydroxide forms a precipitate that sinks to the bottom of the tank, where it is removed.
In the third stage, the aluminum hydroxide is heated to 980 oC, which forms aluminum oxide according to the following reaction:
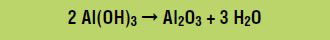
The aluminum is then produced from the aluminum oxide through a technique called aluminum smelting. It is based on a process known as electrolysis, in which an electrical current is used to produce the constituent elements from a chemical compound. In this case, aluminum oxide is separated into aluminum and oxygen (Fig. 2).
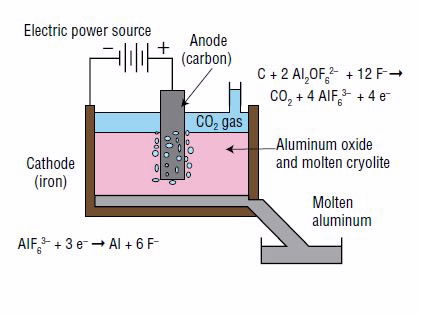
Electrolysis can be performed only on a liquid. Because aluminum oxide does not dissolve in water, it is dissolved in a vat of molten cryolite (Na3AlF6), which consists of the ions Na+ and AlF63–. Aluminum oxide dissolves into two ions, Al2OF62– and F–, by reacting with some of the AlF63– ions from molten cryolite, as follows:

Aluminum (Al) is produced at the negative electrode, called the cathode, according to the following reduction reaction (gain of electrons):
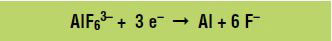
At the positive electrode, called the anode, the Al2OF62– ions react with the anode (which is made of carbon) to form carbon dioxide and carbon monoxide, for example by the following oxidation reaction (loss of electrons):
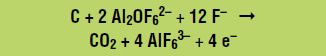
Combining the aluminum oxide dissolution reaction and the oxidation and reduction reactions gives the overall net reaction:
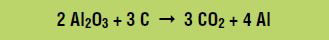
The next step consists of pouring molten aluminum into molds and letting the aluminum solidify into shape. These blocks of solid aluminum, or ingots, are forced through several rollers to become sheets that are less than 1-inch thick. The aluminum sheets are cut to make cans, which are sent to soft drink companies that fill and seal them. The canned soft drinks are sold to consumers who drink them.
In the Western world, the cans are usually collected and sent to a recycling center, where they are cleaned, sorted, and crushed. Then, they go to an aluminum manufacturing plant, where they are shredded, remelted and solidified again.
In India, the cans are collected from garbage dumps and sent to small private companies, where people such as Hamed clean them using acid at room temperature and normal pressure. Then, they melt the aluminum cans in a furnace at 660 oC and pour the molten aluminum into a mold, which later solidify into shape.
Recycling aluminum is much cheaper than extracting it. Aluminum extraction requires temperatures of 1,000 oC and a lot of energy. Additional material—such as cryolite and sodium hydroxide—need to be used, and a significant amount of electricity is needed.
Advantages of recycling aluminum
The key benefit of recycling is that it reduces the amount of waste that needs to be buried or burned. In the case of aluminum, there is also another advantage. If old soda cans were simply buried, new cans would have to be made from new aluminum that would have to come from aluminum ore. So, recycling aluminum has an economic advantage as well as an environmental one.
Not all materials are economically advantageous to recycle. Plastic, for example, is often cheaper to produce from raw materials than it is to recycle. So, while plastic is endlessly recyclable, it is often burned or simply buried because it is not cost-effective to recycle it.
Other constraints also make materials, such as green glass, less desirable to recycle. It is not that the glass cannot be endlessly recycled, it is because the color of glass cannot be changed—once it is green, it will be green forever. A lot of green glass is imported into the United States, containing foreign-made beverages such as wine and beer. But few products are manufactured from green glass in the United States, meaning that most of it is not recycled.
Another reason aluminum is easier to recycle is because it does not corrode, unlike other metals such as iron. If left unprotected, iron is transformed by the corrosive process of rusting into iron oxide. When that happens, the metal has to undergo expensive treatment to remove the oxygen while aluminum, which does not corrode, can simply be melted and molded into a new product.
Changing lives
Thanks to Dharavi residents such as Hamed, a high proportion of aluminum is recycled in India. If it were not for their efforts, nothing would be recycled at all. A big difference between India and the United States is that in India, there is no government recycling program. Indians do not have to separate their waste, while U.S. residents are encouraged to do so.
Hundreds of thousands of Indians forage for recyclable materials in public trash cans. Doing this work exposes them to several health risks because they are working in an unclean environment. Recycling plant workers such as Hamed also face health risks. They are not provided with any safety equipment, even though they work near a furnace that has a temperature of 660 oC. Hamed, for example, works 11 hours per day, enjoys very little time off, and has no chance of attending school. Although Hamed and others are working in the most appalling conditions, the alternative is having no way to make a living.
In the United States, recycling feels like a good habit that some of us try to adopt. In India, it is a lifeline for people struggling to survive. There, aluminum recycling has helped transform local economies, which serves to remind us that recycling not only can improve the environment but can change lives as well.
Selected references
- Sustainability and Recycling, The International Aluminium Institute
- The Informal Recycling Sector in Developing Countries, Gridlines
Tom Husband is a science writer and chemistry teacher in Cambridge, United Kingdom. His latest ChemMatters article, “Recycling to Survive,” appeared in the February 2011 issue.
Also in this Issue (April 2012)

Lighten Up!
What are the advantages and potential drawbacks of compact fluorescent light bulbs over regular light bulbs?

Recycling Aluminum: A Way of Life or a Lifestyle?
In the Western world, recycling aluminum helps to protect the environment. In India, it is a means of livelihood for thousands of people. Learn how recycling aluminum is changing lives and helping the environment.

