Attack of the Gluten
By Margaret Hill
ChemMatters February 2012
Download article
Teacher’s Guide
WORD │ PDF
You wouldn’t know it if you looked at her now, but a year ago, Amy was a mess. She had skin rashes and stomach cramps that would mysteriously come and go. Every time she had a flare-up, Amy suffered in silence, thinking that the skin rashes and stomach cramps were temporary. When they continued, she decided to tell her parents. Medical tests revealed that Amy has celiac disease, a condition caused by an abnormal sensitivity to gluten in the diet.
Unfortunately, gluten is found in many foods. With the help of a nutritionist, Amy and her mother have been working on removing gluten from her diet. It has not been easy because gluten is found in wheat, rye, barley, and oats. Amy misses some of her favorite foods, but she feels better and has fewer skin reactions than before. Amy is relieved that by eating gluten-free foods, she can avoid much of the distress she felt last year. What is gluten and why did Amy’s body react so negatively to it?
Keeping It Together
Figure 1.
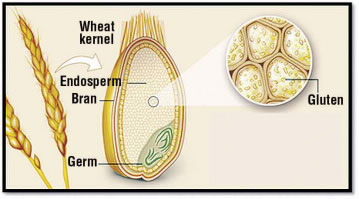
Gluten is a substance found in the grains of wheat and related plants, including barley and rye. It is present in a part of the grain called the endosperm (Fig. 1). Cereal grains are one of the main ingredients in flour, which is often used in household baking because gluten gives elasticity to dough and helps it to rise.
Without gluten, a loaf of baked bread would be flat and dense—not light and fluffy. How does gluten work? Let’s look at what happens when bread dough bakes in the oven. Bread dough is made by mixing flour, water, sugar (sucrose), and yeast—tiny single-celled organisms that feed on sugar in a process called fermentation. But these ingredients are mixed in a certain order, and the resulting dough must be kneaded.
Kneading consists of pressing and folding dough to give it a smoother consistency. During this process, the gluten in the flour becomes elastic and helps the bread to rise and set. The dough rises as a result of yeast fermentation, which consists of several sequential chemical reactions that can be summarized as follows:
C6H12O6 ➔ 2 CH3CH2OH + 2 CO2
(Glucose ➔ Ethanol + Carbon dioxide)
The carbon dioxide causes the dough to increase in volume and rise, but this increase in volume occurs only because gluten supports the dough surrounding the pockets of carbon dioxide. The ethanol evaporates when bread is baked in the oven.

Gliadin and Glutenin
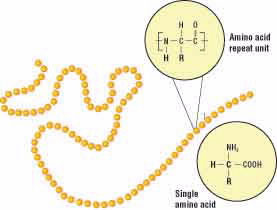
At the molecular level, gluten is made up of two proteins called gliadin and glutenin. A protein is a molecule made of a chain of repeating units called amino acids (Fig. 2). Although an amino acid chain is a linear polymer, it can coil and fold on itself to form a three-dimensional shape. Gliadin usually folds to form a compact spherical shape, while glutenin often assumes a more elongated rope-like shape (Fig. 3).
Figure 2.
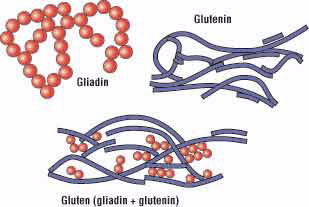
Figure 3.
Amino acids present in both gliadin and glutenin help the two proteins to form hydrogen bonds with each other. According to Arthur Tatham, professor of food science and nutrition at the University of Wales Institute, Cardiff, United Kingdom, gliadin and glutenin form a network within raised dough that allows the dough to keep its shape during baking. This network between the two types of proteins is formed when the bread dough is kneaded, which has the effect of stretching out the glutenin proteins and aligning them with one another. New bonds are made between gliadin and glutenin molecules that preserve this new shape and reinforce the entire protein network.
When the dough is baked, the gluten network continues to support the dough, allowing the bread to emerge from the oven as a light and fluffy loaf. The gluten network in bread consists of aligned and elongated strands that help expand the internal structure of bread. In some ways, this network is similar to the rigid support poles that support a tent frame.
Gluten Sensitivity
Why does Amy’s body react negatively to gluten? The immune system of people with celiac disease mistakenly identifies parts of gliadin and glutenin as foreign and destroys them. The immune system usually looks for “foreign” epitopes—parts of proteins that do not belong to our bodies. Once such epitopes are found, specialized cells from the immune system, called lymphocytes, destroy the foreign proteins.
Epitopes (Fig. 4) usually contain between eight and 11 amino acids. Normally during digestion, the digestive enzymes within the small intestine break up proteins into single amino acids and small chains of amino acids. This is necessary because the intestine can only absorb single amino acids or, at most, chains of three to four amino acids.
Figure 4.
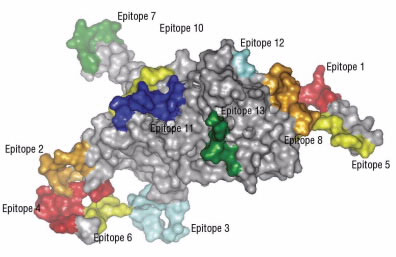
Single amino acids and small chains of amino acids do not cause problems for the intestine nor would they be expected to cause problems with the immune system because lymphocytes are looking for longer chains. It appears, however, that gliadin is not completely broken up by intestinal enzymes. Several longer chains of amino acids remain intact.
Somehow, these larger chains enter the cells lining the intestine, and one of them attaches to an enzyme inside the cells. In people with celiac disease, the complex of the chain of amino acids and enzyme sets off an immune reaction that attacks the complex and, at the same time, damages the intestinal cells.
In the case of Amy—and others with celiac disease—the immune reaction starts when partially digested gluten goes from Amy’s intestine to her bloodstream. There, the epitopes trigger a series of reactions that lead to the release of small molecules called cytokines. Cytokines cause the cells lining the intestine to have increased blood flow—a condition known as inflammation.
Normally, inflammation is a good thing because the increased blood flow allows faster delivery of materials for destruction of the invading bacteria and a means for their quick removal. The problem is that inflammation of the intestinal lining does not stop as Amy continues to eat foods containing gluten. If inflammation goes on for weeks and months, Amy’s intestinal cells become damaged. As a result, she suffers from abdominal pain, diarrhea, and skin rashes.
Longer term exposure over years can result in intestinal damage. The damage can be so severe that her intestinal lining can no longer absorb nutrients. The most effective solution for anyone with gluten sensitivity is to avoid gluten completely. But that’s not easy. Sticking to a gluten-free diet is a challenge, especially because modern foods are heavily based on wheat. From baked goods to frozen pizza dough to boxed cereals and packaged noodles, products containing wheat flour can be found in nearly every aisle of our grocery stores.

People who have celiac disease eat foods that contain wheat substitutes such as sorghum and additives, such as hydroxymethylcellulose and xanthan gum, which help give structure to dough. But these gluten-free foods do not cover the range of foods available to everyone else. Scientists are trying to expand the food choices available to people with gluten sensitivities to provide them with diets that are both nutritious and satisfying. In the meantime, people with celiac disease can choose from a number of gluten-free foods that are available in grocery stores. You may want to explore your neighborhood grocery store and try out some of these foods. You may discover substances you have never heard of and may experience new tastes!
Observing Gluten at Work
To see gluten at work, try this experiment:

Part 1
1. Mix 2 cups of flour with 2 teaspoons of yeast, and 2 teaspoons of sugar
2. Add warm tap water, a small amount at a time, as you knead the ingredients together until you have a nice dough (you will need 1 to 2 cups of water)
Can you feel changes in the mixture as you knead? The dough takes on a stretchy, pliable texture as the gluten network forms.

Part 2
1. Place the entire sample on an electronic balance and record its mass.
2. Then place it in a warm spot for several hours.
3. When you return, you should observe that the dough increased in size.
A casual observer might conclude that its mass also increased. Did the dough mass increase? You can check by making a second mass measurement.
Also in this issue (February 2012)
24 Hours: Your Food on the Move!
What happens to food after we eat it? We usually don’t think about it, but our body does an amazing job at keeping the nutrients that we need and throwing away the food parts that we don’t.

A Tan – Quick, Easy, and Safe?
What is the best way to get a tan? We look at the various options—sun tan, tanning beds, and tanning creams and lotions—and discuss their advantages and potential safety issues.

