Demystifying Gross Stuff
By Brian Rohrig
ChemMatters, October 2011
Here is some good news for you: You can blame the sounds and odors that come from your body on bacteria. Yes, these little critters—which live on our skin, in our mouths, and in our guts—are the ones responsible for a lot of what is going on inside our bodies. Since today’s society is quite obsessed with cleanliness, we tend to be a little uptight about all these bodily sounds and smells. But understanding the science behind what may appear to be so gross may make it, well… less gross.
What’s on your nose?
Probably every teenager has looked in the mirror first thing in the morning to discover a ginormous zit staring back at them. And it usually makes its appearance at the worst possible time, such as the day of the prom.
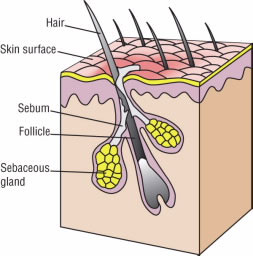
Your skin is porous—it is filled with millions and millions of tiny little holes, or pores. Hair grows out of pores known as follicles. In the skin, glands release an oily substance that is pale yellow and that lubricates and protects the skin (Fig. 1).
Figure 1.
Sebaceous glands in the skin release an oily substance called sebum that lubricates and protects the skin.
This oily substance, called sebum, is one of the main causes of acne. Although it is essential to keep our skin soft and pliable and our hair shiny, too much of it can be a problem. Especially in teenagers, large levels of a sex hormone, called testosterone, are produced, which causes the skin to release a lot of sebum, too. Sometimes, this excess sebum can clog up the pores.
Adding to the mix are dead skin cells. About 30,000 skin cells are shed every minute! A lot of these skin cells are shed inside the pores themselves.
A blocked skin pore also contains bacteria. They feed off the dead skin cells and the clogged sebum within the pores and produce toxins that damage the lining of the pores. As these bacteria grow and multiply, they invade the area surrounding the pore, which can lead to a bacterial infection.
A blocked pore initially turns red because blood rushes to the site, which is one of the ways our body responds to an infection. Then, white blood cells—a type of blood cell responsible for fighting infection—destroy bacteria, build up below the surface of the skin, and die.
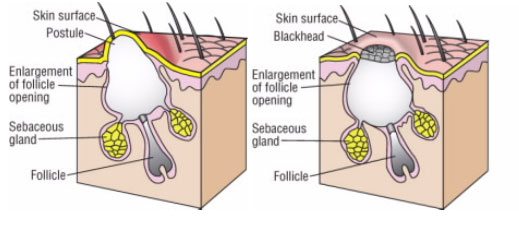
These dead white blood cells, along with dead skin cells and some bacteria, form a white liquid known as pus. A pimple forms when the excess sebum and dead skin cells clog up and block the opening of the pore. This type of pimple is called a whitehead (Fig. 2a).
Another type of pimple, called a blackhead (Fig. 2b), appears when sebum and dead skin cells clog the pore but not the opening, as in a whitehead. While the pore is clogged, its surface remains open. A blackhead appears black because melanin in the dead skin cells reacts with oxygen from the air, which changes the melanin’s color from brown to black.
Figure 2.
Two types of pimples: (a) a whitehead; and (b) a blackhead (respectively)
If the infection worsens, a painful cyst may develop under the skin. A cyst is a fluid-filled sac that is the most severe type of acne. It can cause permanent scarring.
A majority of teenagers have acne, some worse than others. Washing your face with soap and water several times each day is a good way to minimize acne.
Soap is an amphiphilic molecule: One end of the molecule binds to water molecules, while the other end binds to fat molecules, such as the ones present in sebum. These ends are called “water-loving” and “oil-loving,” respectively—hence the word “amphiphilic” (from the Greek “amphis,” meaning “both” and “philia,” meaning “love”).
The reason some molecules bind to water and others to oil—and sometimes to both—is due to one key difference between water and oil, called polarity. Water is polar; oil is nonpolar.
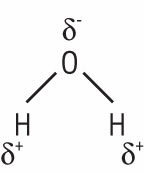
A polar molecule contains regions of partial positive and negative charge that are due to the uneven distribution of electrical charge. In the case of a water molecule, the oxygen end has a greater concentration of electrons than the two hydrogen ends because oxygen tends to attract shared electrons while hydrogen tends to lose them. So, even though the water molecule is electrically neutral, it contains a partial negative charge on the oxygen end and a partial positive charge on each of the hydrogen ends (Fig. 3).
Figure 3.
In a water molecule, the electrons are shared unevenly between the oxygen end and the two hydrogen ends, creating a partial negative charge on the oxygen end and a partial negative charge on the hydrogen ends.
A nonpolar molecule has an even distribution of electric charge, so it has no regions of partial positive and negative charges. This is the case with an oil molecule, in which the electrons are shared equally between the molecule’s atoms.
Polar molecules bind to other polar molecules or to ions. Nonpolar molecules bind to other nonpolar molecules. Polar and nonpolar molecules cannot bind with each other because one has a partial electric charge but not the other.
A common component of soap is sodium stearate [CH3–(CH2)16–COONa]. When this molecule dissolves in water, it produces a stearate anion (C17 H35COO– ) and a sodium cation (Na+ ). The stearate anion is amphiphilic: It has a negatively charged end and a long, nonpolar tail (Fig. 4).
Figure 4.
When dissolved in water, sodium stearate—the soap that most of us use—decomposes into an anion (top) and a cation (Na+) (not shown). The anion has a polar head that binds to water and a nonpolar tail that binds to the skin’s oily substance. It is this anion that helps clean pimples by washing away the skin’s oily substance.

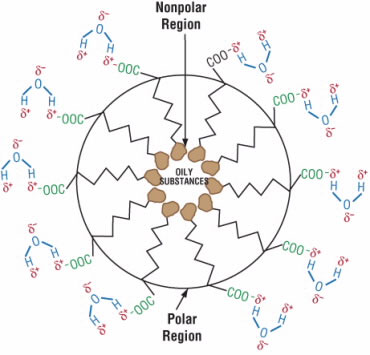
The negatively charged end binds to the electrically positive regions of water molecules, and the long tail binds to sebum molecules. Then, the soap molecules group together to form tiny spheres called micelles (Fig. 5). A micelle consists on the outside of the negatively charged ends attached to water molecules and on the inside of the nonpolar tails attached to sebum molecules. So, the sebum molecules are essentially stuck inside the micelles and can be washed away.
But too much washing can dry out the skin, causing the skin to produce more sebum. Also, once acne has developed, no amount of washing will remove it. At that point, acne is due to a bacterial infection, and only antibacterial agents can treat it. Antibacterial substances destroy or prevent the growth of bacteria. They are available in the form of over the counter creams in pharmacies or are prescribed by a dermatologist.
Figure 5.
After binding to oily substances from the skin, sodium stearate anions pack together to form a sphere called a micelle (top). The negatively charged ends are in contact with water while the long tails trap the oily substances inside the micelle.
Smelly breath
If you wake up and are obsessing over that zit on the end of your nose, you may not even notice a potentially more offensive problem: bad breath. Your mouth is actually teeming with about 10 billion bacteria that take in food and excrete waste. Bacterial waste is in the form of gas, which can be pretty smelly. Bad breath is caused by the combined waste products of these bacteria.
While you sleep, your body stops producing saliva, which contains antibacterial compounds. In the absence of this cleansing liquid, bacteria multiply like crazy at night, and your breath can smell pretty bad in the morning.
During the day, your body produces up to a liter and a half of saliva, which keeps bacteria in check and washes away food particles that may be lodged in your teeth. If you don’t brush your teeth before going to bed, the bacteria have a lot more food particles to munch on at night, leading to a lot of bacterial waste.
One of the best ways you can prevent breath odor—and strengthen your teeth—is by using mouthwash, which kills the bacteria in your mouth. One key ingredient in many mouthwashes is fluoride, which is known to strengthen tooth enamel (Fig. 6).
Fluoride (F– ) is the ionic form of fluorine. It forms when a fluorine atom gains an electron. Fluoride does not exist by itself, but it can be found in compounds, such as sodium fluoride (NaF), which is present in many toothpastes and mouthwashes. When this compound is dissolved in water, the fluoride ions are free to move.
Figure 6. Enamel is the hard white sustance that covers a tooth.
Fluoride ions prevent tooth decay by strengthening the enamel.The primary compound found in tooth enamel is a strong, insoluble mineral called Hydroxyapatite [Ca5(PO4)3(OH )]. Hydroxyapatite contains positive ions (Ca2+) and negative ions (PO43– and OH– ), which are attracted to each other to form the crystalline structure of hydroxyapatite.
The bacteria present on our teeth produce acids that cause hydroxyapatite to break apart—a process called demineralization:

A certain amount of demineralization is normal. But it is also normal for the reverse process, remineralization, to occur:

If too much bacterial acid is produced, demineralization can outstrip mineralization, leading to a cavity. How does this happen? When acids are present in a solution, they dissolve to produce hydrogen ions (H+). In the mouth, as bacteria produce acids, the amount of hydrogen ions builds up. These ions combine with the hydroxide ions produced during demineralization to form water:
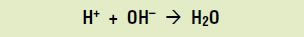
But hydroxide ions are essential to remineralization, so their neutralization by hydrogen ions causes remineralization to slow down. The hydroxyapatite on the surface of the teeth keeps dissolving, ultimately leading to tooth decay.
Fluoride ions present in mouthwashes help the enamel to remineralize. They accumulate on the surface of the enamel, thus creating a barrier that prevents bacterial acids from reaching the enamel. Also, the fluoride ions attract calcium ions, ultimately changing hydroxyapatite into fluoroapatite [Ca5(PO4)3F], which is stronger than the original hydroxyapatite.
Bad breath can be caused by many different gases, but two of the most common ones are hydrogen sulfide (H2S) and methyl mercaptan (CH3SH)—both sulfur-containing compounds. Other gases that lead to bad breath are indole (C8H7N) and skatole (C9H9N), the two gases primarily responsible for the smell of feces.
Passing gas
Eating a lot of fiber can have an undesirable side effect: the production of large amounts of intestinal gas. When this gas is released by the body, it is known as flatulence. The gas itself is known as flatus. “Passing gas” is actually a good way to describe this process. People pass gas 14 times per day, on average. This gas is produced by bacteria in the colon.
Figure 7. Chemical structure of cellulose
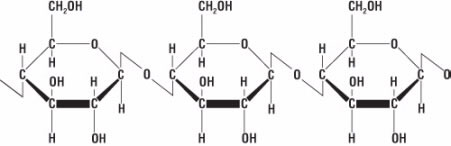
Fiber is made of a substance called cellulose (Fig. 7).Cellulose belongs to a group of materials called carbohydrates that are composed of carbon, hydrogen, and oxygen and are made of a series of repeating small molecules. In the case of cellulose, the repeating small molecule is glucose (C6H12O6) (Fig. 8).
Figure 8. Chemical structure of glucose

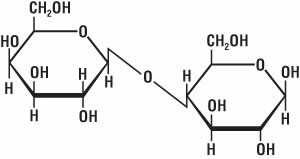
In the colon, bacteria break down cellulose, so if undigested food enters the colon, there is more for the bacteria to feed on. And when you have a lot of bacteria, you have a lot of their waste products in the form of gas. Foods high in fiber—such as fruits, vegetables, and beans—tend to produce a lot of flatulence. Some indigestible sugars can have the same effect. For instance, lactose in milk, which is a carbohydrate molecule (Fig. 9) formed from glucose and galactose molecules, is sometimes not broken down completely. So, dairy products can produce a lot of flatulence, especially if a person is lactose intolerant.
Figure 9. Chemical structure of lactose
Once the basic chemical reactions of the body are understood, the “gross” things of your body won’t seem all that gross. On a molecular level, no one compound is grosser than any other. It’s all just chemistry!
Selected References
- Masoff, J. Oh, Yuck! The Encyclopedia of Everything Nasty, Workman Publishing: New York, 2000.
- Bad Breath: Unusual Causes of Halitosis. Health Tree: http://www.tree.com/health/halitosis-bad-breath-causes.aspx [accessed July 2011].
- What Causes Acne? Skin Care Physicians, April 14, 2010: http://www.skincarephysicians.com/acnenet/acne.html [accessed July 2011].
Brian Rohrig teaches chemistry at Jonathan Alder High School in Plain City (near Columbus), Ohio. His most recent ChemMatters article, “Myths: Chemistry Tells the Truth,” appeared in the December 2010 issue.
Bad Breath
Acne
Also in this Issue (October 2011)

Harnessing Solar Power
In the United States, the main source of energy is fossil fuels, but solar energy is a promising alternative. How do we collect this energy and use
it to produce electricity?

Students Build Solar Homes
Every other year, students compete to build the world’s best solar homes. Check out what students have achieved in 2009 and what they will showcase this year at the Solar Decathlon in Washington, D.C.

Lithium-Ion Batteries: A Clean Source of Energy?
Lithium-ion batteries power computers, iPods, and hybrid cars. But where does lithium come from? Are these batteries safe for the environment?
Subscribe to receive full issue!

