Drivers, Start Your (Electric) Engines
By Michael Tinnesand Feb 2013

Scientists are not particularly known for their sense of humor or playfulness, so it was a bit of a surprise when a participant at the United Nations Conference on Sustainable Development in Rio de Janeiro, Brazil, decided to have a little fun. He drove his 100% electric car into a local gas station and asked for a fill-up. An alliance of electric car manufacturers provided the car to shuttle delegates at the meeting to various sites.
The gas station attendants were totally confused by the lack of any opening for adding gasoline. When they opened the round lid at the front of the car, all they found were terminals. Looking under the hood left them scratching their heads. What they didn’t know is that they may have been looking at the car of the future.
The mechanical systems under the hood did not look all that different from a gasoline-powered version of similar size. But what was missing was notable: no pistons, no crankshaft, no valves, and no variable speed transmission. This means very few visits to the local garage for maintenance, such as oil changes, valve adjustments, or timing chain replacements. In fact, tire rotations are the major item on most of the lists of scheduled maintenance. Since the brakes are part of the energy recovery system on electric cars, they tend to work much longer than conventional gas car brakes, perhaps lasting for 100,000 miles!
Plug-in cars

My wife and I purchased an electric car in November 2011. Nine months later, we had logged about 3,000 miles. We were lucky because Oregon, the state we live in, is one of nine states to receive support for the deployment of electric vehicles. This allowed us to get a grant for an in-home 220-volt charger. It also allowed our hometown to install 34 charging stations and there are nearly 150 in our greater metropolitan area. Last month my total cost for electricity to charge our car at home was $5. It’s estimated the cars can go 70 to 100 miles on less than a $2 charge—the current cost of a half-gallon of gasoline.
The Nissan Leaf and other electric cars—those that run on electricity only—are primarily designed to be city cars, that is, for driving short commutes, local errands, and trips around town. Trips greater than 50 miles one-way are a problem for us. We begin to exhibit so called “range anxiety.” Running out of gas in a conventional car is no fun, but a call to roadside service providers can result in a quick refill and you are on your way.
If our battery runs down, there is no quick fix for us. Although mobile quick charge units are being developed, today our only alternative is to have our car towed home.
Luckily, the car is very smart, and it does everything it can to help prevent this from happening. On-board computers let us know the state of charge in our batteries and what kind of range of travel we have left. If there is a convenient charging station near our destination, we can always plug in for a while before returning home. The nice thing about this is that these charging stations are free (for now), although some charge a parking fee. On-board navigation helps us find these charging stations.
The first electric cars
It’s not clear who first invented electric cars. Records show a number of early inventors who made working vehicles. As far back as 1828, Hungarian inventor Ányos Jedlik, created a simple electric motor and installed it in a tiny model car. In 1834, a blacksmith from Vermont named Thomas Davenport invented a direct current (DC) electric motor and used it to power a miniature model car that ran around an electrified track.
But production of conventional electric cars didn’t happen until the 1890s. By then, the technology for producing rechargeable batteries had progressed to the point where cars didn’t need to operate on electric tracks. By the early 20th century there were a half-dozen manufacturers, including the Edison Company and Studebaker.
But then, as now, the cars were limited by their driving range and the lack of infrastructure to charge batteries. As better highways were built travelers demanded cars with greater range. Mass production lowered the cost and improved the quality of gasoline-powered vehicles. By the 1930s, electric cars were all but extinct.
The typical battery used in those early cars was the lead-acid battery, the type we still use today for starting engines of gasoline-fueled cars. When the battery is in discharge mode—which provides electricity to a device—the following reaction occurs:
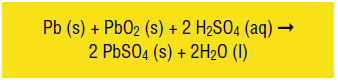
This reaction is a combination of two reactions: an oxidation reaction that occurs at the lead plate of the battery and a reduction reaction that occur at the lead oxide plate of the battery (Fig. 1). At the lead plate, elemental lead is oxidized to lead(II) sulfate, which means that lead goes from an oxidation number of 0 to +2, the result of losing two electrons. These electrons are released to a circuit where they provide the power to run the engine.

The circuit is completed when the electrons travel to the lead oxide plate, where reduction takes place. In this reaction, lead(IV) oxide is reduced to lead(II) sulfate. In the lead(IV) oxide the lead has a +4 oxidation state. In lead(II) sulfate it is +2. This occurs because the reaction adds two electrons to the lead, reducing it from +4 to +2.

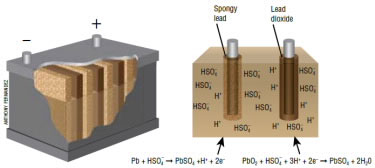
So how do we ever get a battery to recharge? If we pump electrons into the system from some outside source of electricity, we can reverse both of the reactions above. A battery becomes charged when current flows back into it, restoring the chemical difference between the plates. This happens when you are driving without any accessories, and the alternator puts current back into the battery, that is, the current causes the oxidation of lead(II) to lead (IV) and the subsequent release of two electrons.

At least, this is what happens when the batteries are new. Over time, the sulfate ions tend to form insoluble crystals on the surface of the electrodes that do not allow the reverse reactions to occur, and the lead-acid batteries no longer charge.
How the Nissan Leaf works
Lead-acid batteries are too heavy to power the electric cars of today. The state-of-the-art battery for modern electric cars is the type found in the Nissan Leaf. It is a lithium-ion battery pack. Lithium batteries were first proposed in the 1970s, and developed in the 1980s and 1990s to the technology in use today.
The basic chemistry in use is the same as in the lead-acid battery, but with different players and different performance.

Lithium-ion batteries consist of three main components: a positive electrode, a negative electrode and an electrolyte solution. The positive electrode, or cathode, is made from a type of layered lithium oxide, such as lithium cobalt oxide (LiCoO2). The negative electrode, or anode, is made of graphite—a form of pure carbon. The electrolyte is a non-aqueous solution of lithium salt. A permeable membrane, called the separator, plays a critical role between the positive and negative electrodes. This separation of charge is what allows the battery to generate useable electricity. Otherwise it would short-circuit and could explode.
When the battery is used to power a car (discharge mode), lithium ions move out of the graphite anode and cross the electrolyte toward the cathode. In the process, lithium atoms that are intercalated in the graphite are oxidized at the anode, which leaves free electrons behind that can travel through an electric circuit.
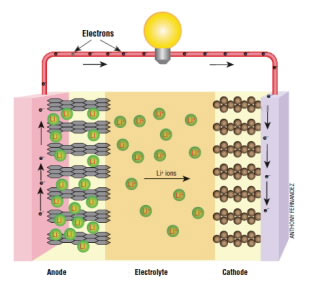
But why is any of this an improvement over the lead-acid battery? For one thing, they are much lighter. Lead is one of the densest common metals, while lithium is one of the lightest. The lithium and carbon electrodes are very lightweight in these batteries.
Another reason is that lithium is a much more reactive metal than lead. This provides a high charge density in lithium-ion batteries. For example, a typical lithium-ion battery can store 150 watt-hours for each 1 kilogram of battery. By comparison, a lead-acid battery only packs 25 watt-hours per kilogram. Another way to think about it is that it would take 6 kilograms of lead-acid batteries to equal 1 kilogram of lithium-ion batteries.
Also, lithium-ion batteries hold their charge much better than other types, losing only 2% per month. They can be charged and discharged thousands of times and they have no “memory effect,” which means you don’t have to completely discharge them before recharging.
The major drawbacks for the electric car seem to be the range, which limits the use of these cars to relatively small distances, its high price, and the fact that most electricity in the United States is generated by burning coal, which leads to environmental pollutants, such as carbon dioxide.
But the range of electric cars will improve over time. The plug-in hybrid Chevrolet Volt combines an electric engine with a gasoline power source, thus eliminating range issues. Also, better-performing lithium-ion batteries are being tested in many laboratories around the world. The best results have shown that such batteries could stay charged for up to 300 miles, in new vehicles such as the Tesla, Model S. If such range, or better, is achieved, and if the cost of electric cars goes down in the future, it looks as if the electric car may regain the popularity that it lost a century ago.
Selected references
- Benefits and Considerations of Electricity as a Vehicle Fuel, U.S. Department of Energy, Fuel Economy.gov. [accessed Nov 2012]
- Powell, D. Old Battery gets a High-Tech Makeover, Science News, July 28, 2012. [accessed Nov 2012].
Michael Tinnesand is a science writer and education consultant who lives in Portland, Ore. His latest ChemMatters article, “The Big Reveal: What’s Behind Nutrition Labels,” appeared in the December 2012 issue.
Also in the February 2013 issue...

Open for Discussion: Teeth Whiteners
How do teeth whiteners work? Are they safe?

Chemical Sudoku Puzzle
Here’s a variation of a SUDOKU puzzle that needs some chemical knowledge as well as logic to solve.

