Graphene: The Next Wonder Material?
By Michael Tinnesand
There is a new wonder material in town that might change our future. Imagine a coffee cup that streams the day’s headlines in real time. Or a cooking pot that can detect the presence of E. coli bacteria that could make you sick. Or a television screen that is as flexible and thin as a piece of paper. All of these applications could be a reality if the wonder material, named graphene, lives up to its hype.
Chicken wire made of carbon
Graphene rocked the world of chemistry in 2004 when scientists discovered that it had remarkable properties: It conducted electricity better than any other common substance, it was the thinnest known material—only one-atom thick—and it was stronger than steel!
After all, carbon is one of the most common and most familiar of the known chemical elements, so scientists were surprised to find that this new form of carbon had such amazing properties.
Carbon comes in many crystalline forms, called allotropes, the most well-known being diamond and graphite. Allotropes are different forms of the same element with different bonding arrangements between atoms, resulting in structures that have different chemical and physical properties. The way atoms are connected to each other in solid materials has a huge impact on their overall properties.
A diamond and a piece of coal are so different that you would never guess that they are both made of the same element—carbon. Diamond is a hard and transparent mineral that is ejected to the surface from deep within the Earth’s interior through volcanic eruptions, while graphite is a black and lightweight material extracted from coal.
In diamond, each carbon atom is connected to four other carbons. This is a very strong arrangement that makes diamond one of the hardest known materials. In graphite, each atom is linked to three others in layers of hexagonal (six-sided) shapes that look like chicken wire (Fig. 2, p. 8). The bonds within the hexagonal sheets are strong, but each layer is only weakly attracted to the next, which allows the layers to slip by one another.
In 2004, Andre Geim and Konstantin Novoselov, two chemists at the University of Manchester, United Kingdom, used this property to produce samples of graphene and discover its remarkable properties. They used sticky tape to separate the layers of carbon in graphite. To get an idea of how their technique worked, think of pressing sticky tape onto a piece of graphite and pulling it away, leaving the sticky surface covered with graphite flakes. Then, press the sticky tape to itself and pull it apart. Repeat, and after a few rounds of this, some flakes on the tape will be only a single one-atom thick layer—pure graphene.

The initial samples of graphene were very small—only a couple of square millimeters in size each—but large enough to test. Because graphene is only one-atom thick, it is considered to be a two-dimensional material, the first example of such a thing in the real world. Despite being the thinnest material known to exist, it is also the strongest material ever tested—100 times stronger than steel.
Even more amazing: Electrons do not scatter as much when they move as they do in other materials, such as silicon. This led researchers to make graphene-based transistors that are twice as fast as traditional silicon transistors, which could make computers run much faster.
Flexible solar panels
Graphene has sparked the interest of engineers who are trying to make new, lightweight, and flexible solar panels that could be used to cover the outside surface of a building, in addition to the roof—which is already being used.
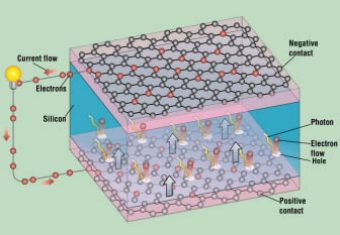
Graphene is nearly transparent to light—not only to visible light but also to other forms of electromagnetic radiation, including ultraviolet and infrared light. Graphene absorbs only 2% of the light falling on it, whether it is ultraviolet, infrared, or all of the wavelengths in between. Combine this with graphene’s ability to conduct electricity, and you have very efficient, electrical conductors that are transparent, thin, flexible, and cheap.
This new type of solar panel is currently under development and consists of organic photovoltaic cells sandwiched between sheets of grapheme (Fig. 1). A photovoltaic cell is a small device that converts the sun’s energy into electricity.
When a photovoltaic cell is sandwiched between two sheets of graphene, light crosses the sheets of graphene and hits the photovoltaic cell. As a result, the photovoltaic cell generates electricity, which is carried by the sheets of graphene.
These lightweight and flexible solar panels could be molded to fit an automobile body or be wrapped around furniture or clothing. When added to any surface, they could collect light and produce electricity.

Foldable cell phones
Until recently, most electronic devices were controlled by pushing buttons, typing on a keyboard, or using a mouse. Today, most cells phones and tablet PCs have touch screens that allow the user to make selections by touching icons or letters directly on the display screen.
The basic idea of how most of these devices work is simple. A layer that stores electrical charge is placed on the glass panel of the screen. When a user touches the screen with his or her finger, or with a stylus pen, some of the charge is transferred to the user, so the charge on the layer decreases. This decrease is measured by sensors located at each corner of the screen, and this information is relayed to a processor inside the device, which determines what kind of action to take.
All of this is possible because these devices use screens that have thin and transparent coatings that are conductive and can hold a charge. Most portable devices today have screens that are coated with a conductive layer made of indium tin oxide. But this material is brittle, so it is layered on glass to protect and support it. This leads to thick and inflexible displays.
Touch screens made with graphene as their conductive element could be printed on thin plastic instead of glass, so they would be light and flexible, which could make cell phones as thin as a piece of paper and foldable enough to slip into a pocket. Also, because of graphene’s incredible strength, these cell phones would be nearly unbreakable. Scientists expect that this type of touch screen will be the first graphene product to appear in the marketplace.
Bionic devices
Because graphene is thin and flexible, it could be integrated into “bionic” devices that would be implanted in living tissue. The term “bionic”—a mix of “biology” and “electronic”—refers to devices that help or improve an organ or tissue, such as artificial hearts or cochlear implants, which assist people with hearing loss.
Graphene is resistant to the salty ionic solutions inside living tissue, so bionic devices made of graphene could have long shelf lives, perhaps lasting a lifetime. This is in contrast to metallic parts that can corrode after a few years, possibly releasing toxic metals into the body.

Also, because graphene conducts electrical signals, it can be connected with neurons, which also send weak electric signals from cell to cell. These electric signals are created when a nerve cell pumps ions—mainly sodium ions (Na+) and potassium ions (K+)—in or out of the cell, causing a difference in electric potential inside and outside the cell.
For example, imagine putting transistors made of graphene along a damaged spinal cord. Such transistors could detect nerve impulses in the undamaged section of the spinal cord and conduct them past the damaged area to the nerves in muscles. This could allow people to regain the use of their arms or legs after a spinal cord injury.
This type of technology could be used to control a mechanical artificial arm or leg. In mechanical limbs, small motors are used instead of muscles to create movement. The graphene bionic device could relay electrical signals to the small motors in an artificial limb, making it move.
You may have noticed that the words “might” and “could” were used many times in this article. That is because there is still a long way to go before any of these applications comes true.
One of the obstacles that needs to be overcome is how to make sheets of graphene large enough and pure enough (containing just carbon) to be useful. Any non-carbon atoms can disrupt the perfect hexagonal pattern for graphene. Many of the samples produced for research are only a few square millimeters in size, but sheets of up to 76 centimeters across have been reported, and breakthroughs seem to emerge every month.
The key is for the layer to be one-atom thick and to have all of its atoms in perfect six-sided rings. This is very difficult to control when producing pure crystals. One commonly used method, called chemical vapor deposition, consists of passing methane (CH4) gas over a sheet of copper. At high temperatures (800 °C–1000 °C), the methane deposits its carbon—ideally in perfect hexagonal sheets—and the hydrogen is released.
In another method, the graphite is dissolved in a solvent and then sprayed in thin layers using inkjet-type printers. The solvent evaporates, and the graphene remains.
But none of these methods have been perfected, as yet. The race is on to be the first to show whether this wonder material can live up to its potential!
Selected references
- Brody, H. “Graphene,” Nature Outlook, Supplement to Nature, March 15, 2012, 483 (7389), March 15, 2012, Supplement pp S29–S44: http://www.nature.com/nature/outlook/graphene/ [accessed Aug 2012].
- Geim, A. K.; Kim, P. Carbon Wonderland, Scientific American, April 2008, 299, pp 90–97: http://www.nature.com/scientificamerican/journal/v298/n4/pdf/scientificamerican0408-90.pdf [accessed Aug 2012].
Michael Tinnesand is a science writer and education consultant who lives in Portland, Ore. His latest ChemMatters article, “A Super Vision for Airport Security,” appeared in the February 2012 issue.
Also in the October 2012 issue...

Open for Discussion: Nanoparticles
Nanoparticles are clusters of a few hundred to a few thousand atoms that promise to change our lives in unique and useful ways.

