In the Fog about Smog: Solving the Smog Puzzle
By Ginger Butcher

Imagine living in Los Angeles in the early 1940s—a fast growing metropolis with endless sunshine, Hollywood celebrities, suburban havens, and fancy cars. Then, imagine waking up one day, and the sun is gone, hidden behind a throat-burning noxious gas. Entire city blocks fade away, blotted out by an unexplainable gas that some thought was part of a World War II attack.
That is exactly what happened in July 1943—and then again and again for more than half a century. Without warning, noxious fog would roll in for days and sometimes weeks, paralyzing the daily activities of Los Angeles residents. Decreased visibility caused fatal collisions of cars and buses; polluted air would spread to the farms beyond the city and damage entire crops in a few hours.
It would take years for scientists to piece together the puzzle of this Los Angeles smog and decades to implement policies to improve the air we all breathe. Today, scientists are able to monitor the pieces of this puzzle from space thanks to NASA’s Aura satellite.
But what is smog? What were the pieces that made up this chemical puzzle? And how did Los Angeles free itself from this toxic nuisance and bring back “sunny California”?
Finding the pieces of the smog puzzle
The term “smog” is a contraction of the words “smoke” and “fog.” Smog usually consists of soot particles, sulfur dioxide, and other compounds. The citizens of Los Angeles initially blamed the pollution on oil refineries and factories, and they were partly right. State and local officials responded by establishing air pollution control offices, commissioning studies, restricting emissions of sulfur dioxide and smoke from power plants and industry, and banning trash-burning in backyards—a common practice at the time.
While these efforts helped reduce air pollution, they did not reduce the occurrence of smog. In October 1954, a series of intense smog events closed schools and industry in Los Angeles for almost a month. Citizens were frustrated at the lack of progress. They wanted breathable air!
Keeping Tabs on Air Pollution from Space
The first piece to the Los Angeles smog puzzle was its smell, which was different from the sulfurous smog that claimed lives in Donora, Pa., and London, England. In those places, the culprit was the burning of coal, but there was very little of that in Los Angeles. Also, something in the Los Angeles smog was destroying rubber tires and damaging crops.
A chemist named Arie “Haagy” Haagen-Smit noticed that the smog in Southern California had a “bleach-like” odor that reminded him of a chemistry lab. In previous research, he used an apparatus to extract flavor compounds from plants to figure out, for instance, what gives pineapples their characteristic smell. Haagy decided to analyze Los Angeles's air with the same apparatus, which indicated the presence of oxidized volatile organic compounds (VOCs). So he exposed smog-sensitive plants to oxidized VOCs. Sure enough, the plants showed damage similar to plants damaged by the Los Angeles smog. Haagy thought he had the answer!
Haagy knew that plenty of VOCs were present in Los Angeles’s air, given the significant petroleum industry in Southern California and the number of cars on the road there. Gasoline is made up of organic compounds, some of which evaporate, or volatilize, in the air. (This is why you can smell gasoline fumes.) The petroleum industry in Los Angeles estimated that 120,000 gallons of gasoline were lost every day to evaporation during the refining process. Cars and trucks were equally inefficient, spewing uncombusted or partially combusted gasoline—containing volatile organic compounds—out of their tailpipes at a rate of 850 tons per day.
But when Haagy created synthetic smog in the lab, he unknowingly introduced another piece to the puzzle when oxidizing the VOCs: ozone. Oxidation is the process of combining a molecule with oxygen, and since ozone (O3) is a highly reactive molecule made of three oxygen atoms, it was the perfect compound to oxidize VOCs for his experiment.
What was oxidizing the VOCs in Los Angeles’s air? Surely, it wasn’t ozone, a powerful oxidant that was not emitted directly by tailpipes or smokestacks. Haagy and other scientists eventually figured out that nitrogen oxides in the air were reacting with the VOCs, and sunlight provided the catalyst for these chemical reactions to create ozone. Nitrogen oxides are produced during the combustion process inside car engines and are released in the exhaust.

The pieces to the puzzle had fallen into place. Ozone has a bleach-like smell, and it destroys plants and rubber products. Los Angeles had plenty of cars to supply nitrogen oxides and VOCs. And the famous California sunshine was the perfect catalyst for the chemical reactions that form ozone.
One piece of the puzzle was still missing: Why did Los Angeles have more of this kind of smog than other major cities? The answer was a matter of geography and topography. Los Angeles is surrounded by mountains, which trap VOCs and nitrogen oxides in the valleys where people live and breathe. By the early 1940s, Los Angeles had all of the ingredients for catastrophic smog events, which plagued the city for more than half a century.
Taming the culprits of smog
Haagy’s findings were published in 1950, and his fellow researchers confirmed in 1955 that ozone from VOCs and nitrogen oxides was at the root of the Los Angeles smog. But when gasoline and cars were identified as the primary pieces of the smog puzzle, researchers pointed a finger at our beloved automobiles, a fundamental part of the American dream.
It took years of politics, policy, and innovation to reduce the emissions of VOCs and nitrogen oxides that make ozone. Enforcement of local laws was difficult because air pollution does not heed city and country borders. Regulations to reduce air pollution quickly became a state, as well as a national, issue and eventually led to the Clean Air Act, passed by Congress in 1970.
While legislators were writing regulations, the petroleum and auto industries were innovating. Oil companies reformulated gasoline to burn more efficiently, reducing the amount of unburned VOCs in car exhaust. Gas stations put sleeves on gas pump nozzles, reducing the amount of VOCs evaporating from gasoline.
Regulations prompted the automobile industry to make more fuel-efficient cars and to develop catalytic converters that reduced the amount of nitrogen oxides and VOCs—along with carbon monoxide—released from cars. Similar technologies, such as selective catalytic reduction devices, were designed to reduce the amount of nitrogen oxides released from power plants.
Tracking puzzle pieces from space
Air pollution is not just confined to Southern California; it is produced wherever people burn fuel for energy. It is dispersed by the wind over long distances, across state and country borders. So, to monitor concentrations and distributions of pollutants, we need a global view. In 2004, NASA launched several remote sensing instruments onboard the Aura satellite that can observe the air quality of the entire planet in just 24 hours.

Aura's Ozone Monitoring Instrument (OMI) measures the amount of ozone in our atmosphere. While ozone is harmful at the ground level, it is beneficial in the stratosphere—the portion of the atmosphere ranging from approximately 10 kilometers (km) to 50 km (6 to 30 miles) above the Earth’s surface—where a layer of ozone absorbs harmful ultraviolet radiation (see article on p. 12). OMI has been essential to studying this protective ozone layer from space.
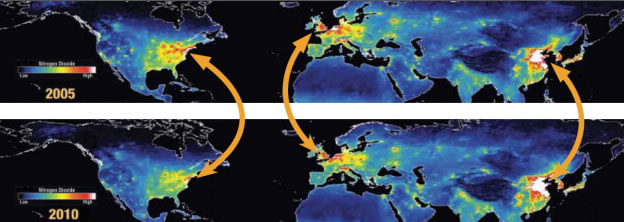
While OMI was not designed to study ground-level ozone, it provides scientists with a global view of a key piece in the smog puzzle—nitrogen dioxide (NO2), a component of nitrogen oxides (NOx). Nitrogen dioxide has a relatively short lifespan, so it is concentrated near the source of its emission. As a result, when OMI detects the presence of nitrogen dioxide in the atmosphere, scientists can identify and monitor its sources.
A global view of nitrogen dioxide reveals high concentrations around cities. A comparison of data collected in 2005 and 2010 shows that levels decreased significantly in the United States and Europe, thanks to policies and regulations to reduce emissions, but that air pollution increased in East Asia.
Monitoring the missing pieces
Although we have made great progress during the past 70 years at reducing pollutants in the air, ground-level ozone is still a problem. Since 1980, the U.S. Environmental Protection Agency (EPA) estimates that ozone levels have decreased by 28%, mainly because the emission of nitrogen oxides was reduced by 52%. But more needs to be done. Although air quality is improving over time, as many as 108 million Americans currently breathe unhealthy levels of ozone, particularly during sunny summer months.
Another problematic air pollutant is particulate matter, tiny particles that can be harmful to our lungs if we breathe them in. Particulate matter is produced during combustion by car engines and power plants. In countries where it is common to burn coal for heat, particulate matter can lead to thick smog, as it did during the winter of 2012–2013. Cities around the world, from Turkey to China, reported increased visits to hospitals for respiratory problems, and government authorities issued warnings for people to stay indoors.
Through continued research and the development of pollution-control technology and renewable energy sources, we can one day make sure these pollutants aren’t produced any more. Until then, we need to monitor air quality with instruments on the ground and on satellites such as Aura. NASA’s unique view of our planet provides the data necessary to help us make better choices and improve the air we breathe.
Ozone and Our Health
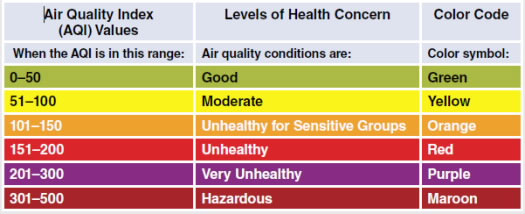
The air quality index is a standardized index of the air quality in a given location. The U.S. Environmental Protection Agency calculates the air quality index for five major air pollutants that can contribute to smog and are regulated by the Clean Air Act: ground-level ozone, particle pollution (also known as particulate matter), carbon monoxide, sulfur dioxide, and nitrogen dioxide.
What do the Air Quality codes mean for us?
During a Code Orange or Red warning for air quality, we are advised to stay indoors to avoid breathing polluted air. On sunny and hot days, ground-level ozone can be high, particularly in the afternoon. We are asked to reduce driving and pump gas early in the morning or at night to reduce the amount of volatile organic compounds and nitrogen oxides emitted during daylight hours, when sunlight initiates the chemical reactions that form ozone.
Factoid: What Is a Volatile Organic Compound?
A volatile organic compound (VOC) is an organic chemical compound that can evaporate under normal conditions of temperature and pressure. There are thousands of VOCs, including man-made sources, such as gasoline fumes, and natural sources, such as pine scent from pine trees.
Selected references
- Our Changing Atmosphere: Discoveries from EOS Aura, Goddard Space Flight Center, NASA, 2010: http://aura.gsfc.nasa.gov/images/AuraBrochure2010opt2.pdf [accessed Feb 2013].
- Air Quality Index—A Guide to Air Quality and Your Health, AIRNow, Environmental Protection Agency, Dec 9, 2011: http://www.airnow.gov/index.cfm?action=aqibasics.index [accessed Feb 2013].
Ginger Butcher is the Education and Public Outreach lead for NASA's Aura Mission. She has worked at NASA for more than 15 years and has published a variety of educational products that explore NASA science, including NASA's Tour of the Electromagnetic Spectrum and the Adventures of Echo the Bat. Visit www.aura.gsfc.nasa.gov/outreach to learn more about NASA science and the Aura mission.
Also in the April 2013 issue
Miles above the surface of the Earth, a thin layer of ozone gas acts as a shield that protects us from harmful ultraviolet light. But chemicals released in the atmosphere have caused a huge hole in the ozone layer above Antarctica.

Goodbye Plastic, Hello Edible Wrappers--or Nothing at All!
Have you ever considered eating your food wrappings? Now, it is possible,and it is a nice way to protect the environment.

