Ice, Cream... and Chemistry
By Brian Rohrig February 2014
There is perhaps no fonder childhood memory than the local ice cream truck driving through the neighborhood, music blaring from its tinny speakers, beckoning all to partake of its frosty delights. But ice cream is not just for kids. U.S. residents consume 1.5 billion gallons of ice cream each year; that’s roughly 5 gallons (19 liters) per person! The ice cream we all enjoy is the result of years of experimentation involving—you guessed it—chemistry!
Air is Important
If you have ever made ice cream, you already know what goes into it, ingredients such as milk, cream, and sugar. But there is one main ingredient that you may not have thought about, probably because you can’t see it—air.
Why is air so important? If you have ever had a bowl of ice cream melt, and then refroze it and tried to eat it later, it probably did not taste very good. If you set a whole carton of ice cream on the table and let it melt, the volume of the ice cream would simply go down. Air makes up anywhere from 30% to 50% of the total volume of ice cream.
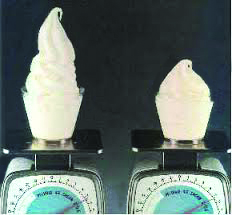
To get an idea of the effect of air on ice cream, think of whipped cream. If you whip air into cream, you get whipped cream. Whipped cream has a different texture and taste than plain cream. Plain cream tastes sweeter than whipped cream. Just like ice cream without air, pure cream has a sickly, overly sweet taste. This is because the structure of a substance can have a big effect on how it tastes, and that the structure often controls the rate at which flavor molecules are released into the mouth. The larger the structure (ice cream, in this case), the longer it takes for the flavor molecules to be released. Flavor molecules that trigger receptors on the mouth and tongue.
The amount of air added to ice cream is known as overrun. If the volume of ice cream is doubled by adding air, then the overrun is 100%, which is the maximum allowable amount of air that can be added to commercial ice cream. The less expensive brands usually contain more air than the premium brands. One side effect of adding a lot of air to ice cream is that it tends to melt more quickly than ice cream with less air.
The amount of air also has a huge effect on the density of ice cream. A gallon (3.8 liters) of ice cream must weigh at least 4.5 pounds, making the minimum density 0.54 gram per milliliter. Better brands have higher densities—up to 0.9 grams per milliliter. The next time you visit a grocery store, compare cheaper and more expensive brands by holding a carton in each hand—you should be able to notice a difference. Then read the net weight on the label to confirm your observation. Due to the high fat content of ice cream, however, and because fat is less dense than water, any ice cream will always be less dense than any aqueous solution, otherwise you would not be able to make root beer floats!
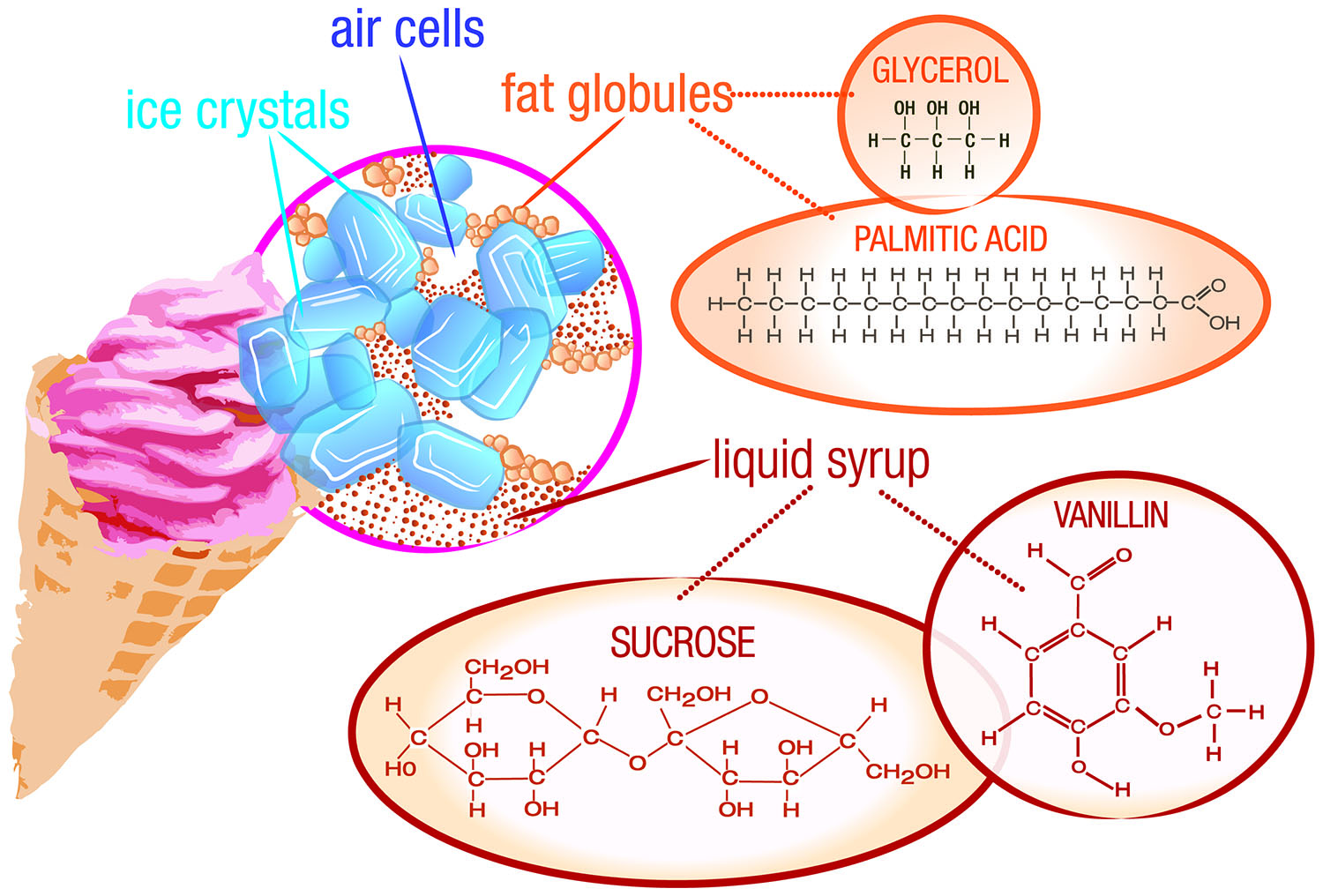
Ice cream is an emulsion—a combination of two liquids that don't normally mix together. Instead, one of the liquids is dispersed throughout the other. In ice cream, liquid particles of fat—called fat globules—are spread throughout a mixture of water, sugar, and ice, along with air bubbles (Fig. 1). If you examine ice cream closely, you can see that the structure is porous. A typical air pocket in ice cream will be about one-tenth of a millimeter across. The presence of air means that ice cream is also a foam. Other examples of foams are whipped cream, marshmallows, and meringue (as in lemon meringue pie).
Sugar and Fat
Milk naturally contains lactose, or milk sugar, which is not very sweet. Ice cream makers need to add a lot more sugar than you probably realize—usually sucrose or glucose. Cold tends to numb the taste buds, making them less sensitive. So more sugar needs to be added to produce the desired effect at the low temperatures in which ice cream is usually served. If you taste ice cream at room temperature it will taste overly sweet. You may have noticed this same effect with carbonated soft drinks. If consumed warm, they taste sickly sweet. In parts of the world where soft drinks are normally consumed warm, there is less added sugar. If these same soft drinks were served cold, they would not taste sweet enough.
A big reason why ice cream tastes so good is because of its high fat content. Unless it is labeled as light, low-fat or non-fat, ice cream must contain at least 10% fat, and this fat must come from milk. (You cannot use lard when making ice cream!) Before milk is homogenized, a thick layer of cream rises to the top. This cream has a high fat concentration—up to 50%—and supplies most of the fat in ice cream.
Premium ice creams may have up to 20% fat, which gives it a velvety, rich texture. Reduced fat ice cream does not taste as good as the real thing, and tends to lack the creamy texture. Although fat is frequently vilified, it has its purpose. Most foods that taste delicious probably contain fat. Fat fills you up, so you don’t have to eat as much to feel full.
The problem with using fat as an ingredient in any food is that it doesn’t mix well with a lot of other substances. Fat is nonpolar, meaning positive and negative charges within the fat molecule are equally dispersed. A polar substance, such as water, has separate regions of positive and negative charge—one end of a polar molecule has a partial positive charge, and the other end has a partial negative charge. Polar and nonpolar substances do not mix. Just like oil floats to the top of water, the fat content in ice cream has a tendency to separate out, as well.

Brain Freeze
When ice cream touches the roof of your mouth, it may trigger a cold headache. The cause is a dilation of blood vessels in your head located above the roof of your mouth. When this nerve center gets cold, it seems to overreact and tries to heat your brain.
Keeping It All Together
Because ice cream is an emulsion, you would expect that the fat droplets that are present in the mixture would separate after some time, similar to a bottle of salad dressing in which the oil separates from the rest of the dressing. When you shake up a bottle of salad dressing, the two parts come together. But after a few minutes, they begin to separate. That’s because the oil droplets interact with one another, a process called coalescence.
In the case of milk, each fat droplet is coated with a layer of milk proteins that prevents the fat droplets from interacting with one another. These milk proteins act as “emulsifiers”— substances that stabilize emulsions and allow the liquid droplets present in the emulsion to remain dispersed, instead of clumping together. Because these milk proteins have a nonpolar side, and because like dissolves like, the nonpolar sides of the proteins are attracted to the nonpolar fat globules. This is good in milk, but not so good in ice cream, in which the fat droplets should coalesce to trap air.
So another emulsifier is added to allow the fat droplets to coalesce. This emulsifier replaces milk proteins on the surface of the fat droplets, leading to a thinner membrane, which is more likely to coalesce during whipping. A common emulsifier is lecithin, found in egg yolks. Lecithin is a generic term that refers to a group of molecules that consist of long chains of fatty acids linked to a glycerol molecule, along with choline and a phosphate group (Fig. 2).
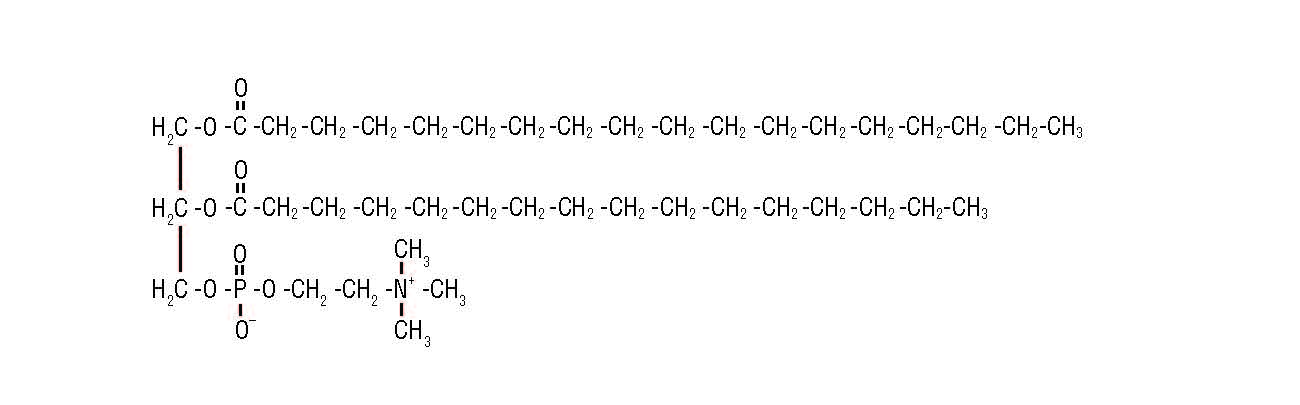
Lecithin inserts itself between the fat globules, which helps the fat globules to clump together and, as a result, the air bubbles that are present in the mix are trapped by this partially coalesced fat. This adds firmness and texture to the ice cream, enabling it to retain its shape.
Closely related to emulsifiers are stabilizers, which make the texture creamy. Stabilizers have two roles: First, they prevent large crystal formation. In the presence of stabilizers, ice cream contains small ice crystals that are easier to disperse and, therefore, they melt more slowly than larger ice crystals would. Second, emulsifiers act like a sponge by absorbing and then locking into place, any liquid in the ice cream.
Common stabilizers are proteins such as gelatin and egg whites. Guar gum, locust bean gum, and xanthan gum can also be used. Look for carrageenan and sodium alginate on the ingredient label of your ice cream container. Both are derived from seaweed! Without these stabilizers, ice cream might look like a milkshake.
Once you get all of the ingredients together in a mixture, you need to freeze the mixture to form ice cream. The dissolved solutes (mostly sugar) in the liquid portion of the mixture lower its freezing point. A freezing point depression of 1.86 °C occurs for every mole of solute added to 1 kilogram (kg) of water. In other words, if you dissolve one mole of sugar in 1 kg of water, water will no longer freeze at 0 °C, but rather will freeze at –1.86 °C.
Freezing point depression is a colligative property, meaning that the effect is observed regardless of the specific identity of the solute—all that matters is how many moles are dissolved. A typical batch of ice cream will freeze at -3 °C (27 °F), due to the presence of all the dissolved solutes.
A recent trend is ice cream made with liquid nitrogen. One shop in San Francisco, Calif., aptly named Smitten Ice Cream, has a viewing area where customers can watch ice cream being made with liquid nitrogen, accompanied by the impressive plume of fog that is released. Liquid nitrogen, which boils at –196 °C, will freeze ice cream almost instantly. Because the ice cream freezes so quickly, the size of the crystals is small, resulting in a creamy texture. And because it boils when it hits the mixture, the ice cream is aerated during the process. The popular Dippin’ Dots are also made using liquid nitrogen. It is no exaggeration to say that ice cream made with liquid nitrogen is the coolest ice cream around!
Types of Ice Cream
Soft-serve ice cream, frozen custard, and frozen yogurt. What is the difference?

Regular ice cream is typically served at –12 °C, while soft-serve ice cream is served at –6 °C. This higher temperature is responsible for a softer product. Soft-serve ice cream, or soft serve, for short, contains less fat and more air than regular ice cream. Soft serve with insufficient air will have a yellowish color. The whiter the soft serve, the better the quality. As ice cream melts, you may have noticed this yellow color, which is simply the actual color of the ingredients used to make it. By adding air and fluffing it up, ice cream is better able to reflect white light, producing the white color. This is because the molecules in ice cream are large enough to reflect visible light (whereas, for example, water molecules are too small to reflect visible light, because the size of a water molecule is smaller than the wavelengths of visible light).
Frozen custard differs from ice cream in that it contains at least 1.4% egg yolks. The egg yolks are made of lecithin, an excellent emulsifier. The result is a product with a smoother, creamier texture. Another difference is that custard contains much less air than ice cream. No air is mixed during its manufacture; instead, air is introduced during mechanical agitation as the frozen custard is being made. It is churned more slowly during its manufacture to minimize the amount of introduced air. Less air leads to a thicker, denser product. Frozen custard is typically made fresh each day in the store. It is frozen quickly to prevent large crystals—of water, lactose, or any added sugar—from forming.


Frozen yogurt is making a huge comeback these days, with self-serve frozen yogurt shops offering a plethora of toppings popping up seemingly on every corner. Frozen yogurt is viewed as a healthier alternative to ice cream, unless you top it off with a generous helping of gummy bears! It does contain less fat, but that means you can eat more without feeling full. And to compensate for less fat, often a lot of sugar is added. The biggest difference is that instead of cream, yogurt is added as the primary dairy product. From there, the process is similar to making regular ice cream.
Selected References
- Gooch, A. “The Chemistry behind Ice Cream.” Chicago Tribune, June 30, 2004: http://articles.chicagotribune.com/2004-06-30/entertainment/0406300068_1_ice-cream-homemade-ice-ice-crystals-form [accessed Dec 2013].
- Halford, B. “Ice Cream: The Finer Points of Physical Chemistry and Flavor Release Make this Favorite Treat so Sweet.” Chemical & Engineering News, Nov 28, 2004: http://pubs.acs.org/cen/whatstuff/stuff/8245icecream.html [accessed Dec 2013].
- Kilara, A.; Chandan, R. C.; Hui, Y. H. “Ice Cream and Frozen Desserts.” Handbook of Food Products Manufacturing, John Wiley Online Library, Chapter 74, pp 593–633, Aug 1, 2006: http://www.researchgate.net/publication/227580162_Ice_Cream_and_Frozen_Desserts/file/9c9605151b162a696c.pdf [accessed Dec 2013].
Brian Rohrig teaches chemistry at Metro Early College High School in Columbus, Ohio. His most recent ChemMatters article, “Hot Peppers: Muy Caliente!” appeared in the December 2013 issue.
Ice Cream–True or False?
Many myths and half-truths are floating around about ice cream. Test your ice cream knowledge by deciding whether each of the following is true or false:
1. Margaret Thatcher, former prime minister of England, helped to develop the formula for soft-serve ice cream before she entered politics, while working as a chemist in the food science industry.
2. Soft-serve ice cream was born on Memorial Day in 1934 when an ice cream salesman broke down and had to sell his melting ice cream, which was a big hit.
3. Modern-day ice cream was accidentally discovered in 1782 by Martha Washington, wife of the first U.S. president, who left a bowl of cream on the back steps of her home one night, and in the morning found it had hardened into ice cream.
4. The infamous Roman emperor Nero had slaves bring ice from the mountains so he could enjoy chilled desserts by pouring fruit juice and honey over the ice.
5. Ice cream sundaes were invented in the late 1800s in New York to circumvent the law prohibiting the serving of ice cream on Sunday, hence the name.
6. Eskimo pies were originally called “I Scream Bars.”
7. Explorer Marco Polo was the first person to bring ice cream to the New World, bringing the recipe from China.
8. The ice cream cone was invented in 1904 during the St. Louis Exposition when an ice cream vendor ran out of bowls and substituted rolled up waffles instead.
9. The ice cream sandwich was invented by Earl of Sandwich, in England.
Answers to True or False
1. Probably false. While Thatcher did work as a chemist for a company that developed soft serve ice cream, her actual role in developing the product was likely minimal, if at all.
2. Likely true, but may not be the first.
3. False.
4. Probably true.
5. Possibly true, but there are several other equally compelling stories about the origin of the sundae.
6. True.
7. Story likely true, but may not be the first.
8. Story likely true, but may not be the first.
9. False. Don’t mistake the ice cream sandwich for the actual sandwich, which was invented by the Earl of Sandwich.
Note: If you are concerned about the ambiguity of these answers, now you know why ice cream historians are still arguing about the origins of ice cream!

