Have you ever noticed that puddles seem to dry up faster on a warm day than on a cool day? Why does that happen and where do you think the water goes?
Here's what to do:
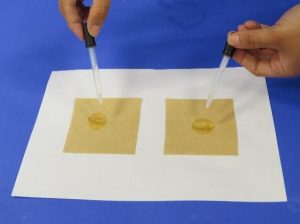
- Use your scissors to cut out two squares of brown coffee filter that are about 10 cm x 10 cm. Put about 1 teaspoon of room temperature water in a small plastic cup.
- Use a dropper to put 1 drop of room temperature water in the center of each piece of coffee filter.
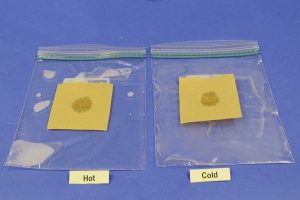
- Put ½ cup of room temperature water into a zip-closing plastic bag. Try to get as much air out as you can and close the bag securely.
- Ask your adult partner to put ½ cup of hot tap water into the other plastic bag. Try to get as much air out as possible and close the bag securely.
- Place one paper on the hot water bag and the other on the cold water bag.
After about 4-5 minutes, take a look at the coffee filters. What do you notice? If there is not much of a difference between them, check again in another 4-5 minutes.
What to expect
The wet spot on the paper on the hot water dries faster than the wet spot on the paper on the cold water.
What's happening in there?
Any sample of water, even a drop or less, is made up of an enormous number of water molecules. Some of the water molecules are moving fast enough to break away from the rest of the water and go up into the air. When water molecules do this, they change from liquid water to water vapor – a gas. This changing from a liquid to a gas is called evaporation. Heating a liquid causes the water molecules to move faster which makes evaporation happen faster. That’s why there is more evaporation from the paper on the hot water than on the colder water.
What else could you try?
Temperature isn’t the only thing that affects evaporation. The type of liquid matters too. You can try an experiment to see if alcohol or water is the faster evaporator.
What you'll need:
- 2 pieces of brown coffee filter (10 cm x 10 cm)
- 2 droppers
- Room temperature water
- Isopropyl alcohol (70%)
Be safe
Be sure to review the safety instructions on page 1 before proceeding.
Here's what to do:
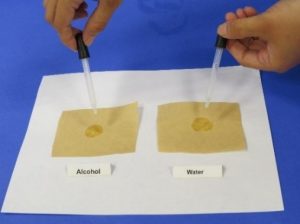
- Put your two pieces of coffee filter on the work surface in front of you.
- You and your adult partner should each use a dropper to place 1 drop of water on one piece of paper and 1 drop of alcohol on the other.
- Allow the liquids to soak in and then pick up the papers and gently wave them to help them dry.
- After about 2 minutes, look at the papers.

What to expect
The alcohol evaporates much faster than the water.
What's happening in there?
Water molecules attract each other and stick together more than alcohol molecules attract and stick to one another.
Think about this …
If water molecules stick together better than alcohol molecules, do you think you can get more water or more alcohol to stay on the surface of a penny?
- You can find out with a quick experiment.
Use a dropper to place drops of water, one-at-a-time on the surface of a penny. Count the drops as you put them on and see how many you can add before the water falls off the penny. - Now, dry off the penny and do the same thing with drops of alcohol.
You were probably able to get way more drops of water on the penny than drops of alcohol. Why do you think that happened?