Kelly Pneumatic Iron Process
National Historic Chemical Landmark
The American Chemical Society, Lyon County Public Library and Murray State University recognized William Kelly's pneumatic process for refining iron as a National Historic Chemical Landmark on May 11, 2015.
The late-nineteenth century was a time of extensive change in business and industry. The expansion of the railroads and manufacturing that followed the Industrial Revolution created great demand for key materials like iron and steel.
In this environment, William Kelly devised a new process for refining iron. Kelly’s invention used chemical reactions to remove impurities in molten pig iron, a crude form of iron, and reduce the fuel requirements of the process. It was a key development that contributed to the vast expansion of the iron and steel industries around the turn of the 20th century.
Early manufacture of iron
Iron is an important material in human history. Iron occurs only rarely as an elemental metal and usually requires processing from ore before it can be used. Since the development of early refining processes around 1200 BCE, cultures have adapted iron for use in weaponry, transportation and other purposes.
In the 1800s, three forms of iron were common: cast iron, wrought iron and steel. The carbon content gives each form its unique properties. Cast iron has a carbon content of 2-4%, making it hard, but brittle. Wrought iron is purest, with only 0.04-0.08% carbon, and is therefore malleable. Steel has carbon content between the two, making it both hard and malleable.
For centuries, iron and steel were produced in small batches by artisan foundry-masters who worked ores and crude foundry products with heat to produce useful metals. Their methods were labor and fuel intensive. Despite the foundry workers’ skills, large-scale production was impossible given the techniques then known. By the mid-1800s, it was clear that manufacturing improvements were needed. Expanded industry—especially railroads—created a vast appetite for metals of high strength that could be molded into useful forms.
To the process of manufacture I gave my first and most serious attention; and, after close observation and study, I conceived the idea that, after the metal was melted, the use of fuel would be unnecessary—that the heat generated by the union of the oxygen of the air with the carbon of the metal would be sufficient to accomplish the refining and decarbonizing of the iron.”
—William Kelly, The Manufacture of Iron in All Ages, 1892
William Kelly's pneumatic iron
William Kelly (1811–1888) was the American ironworks owner who is credited with first identifying a pneumatic process for iron refining. Kelly’s discovery was a critical development in the commercial production of iron.
In 1846, Kelly, of Pittsburgh, Pennsylvania, married Mildred Gracey of Eddyville, Kentucky. The area surrounding Eddyville held numerous ironworks, and Kelly persuaded his brother to join him in purchasing Union Forge on the nearby Cumberland River to produce wrought iron products.
The forge had access to the two main natural resources an ironworks required: iron ore and forests. Because refining required huge amounts of fuel, soon the forests on their property were devastated from being cut for use in the foundry. Recognizing the threat of fuel shortages to the success of their business, Kelly began a series of experiments in the hope of finding a more efficient method of refining iron ore into wrought iron. He experimented at Union Forge in 1847 but stopped shortly thereafter, likely having found little immediate success. He resumed experiments at the firm’s Suwanee Furnace a few miles northwest, starting in 1851.
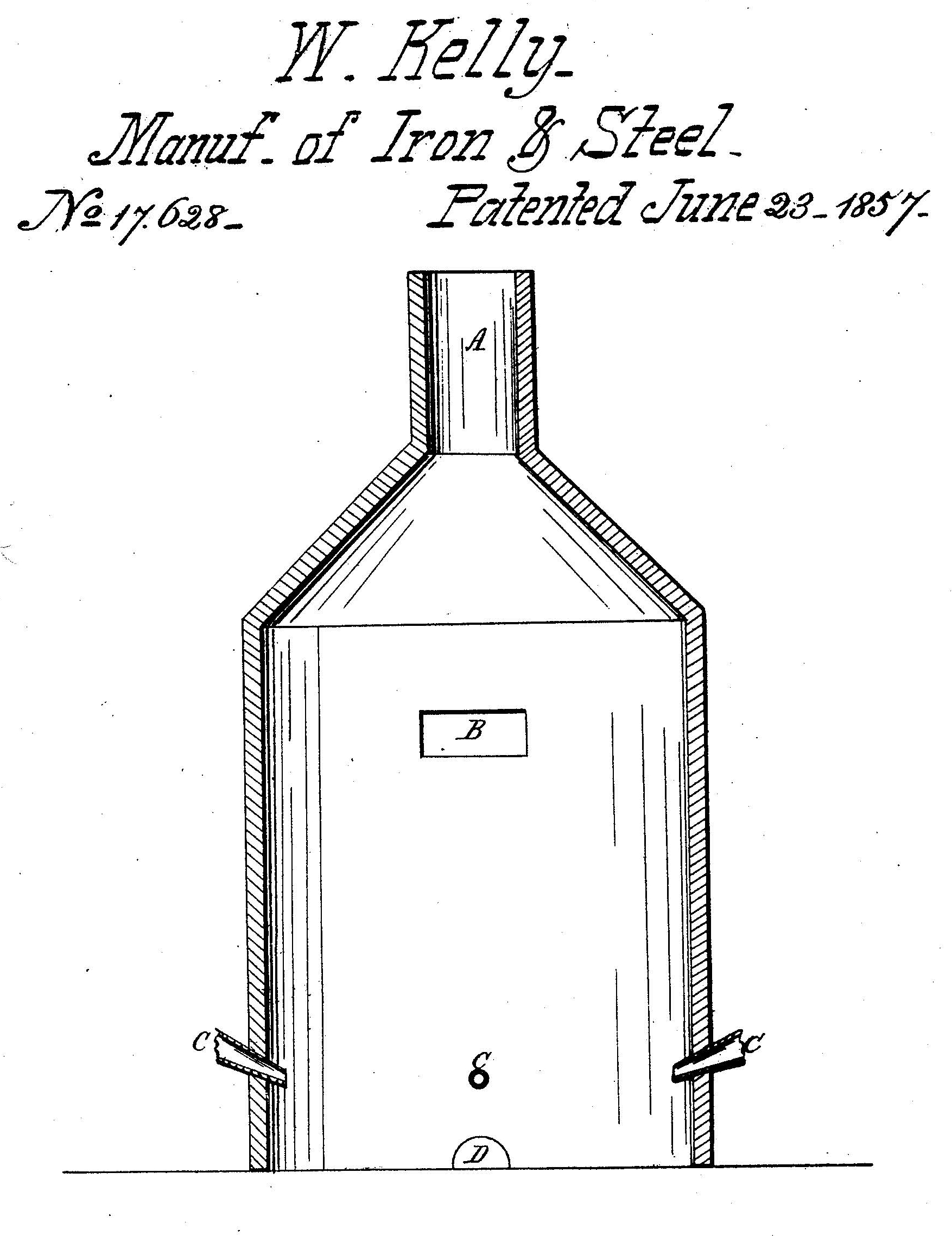
To conduct his experiments, Kelly constructed a specially-designed cupola furnace. Molten pig iron (the initial product of smelting iron ore and a precursor to refined iron) was poured into the cupola, which contained perforations at the bottom called tuyeres. When air was forced through the tuyeres into the molten iron, various chemical reactions occurred. Oxygen in the air combined with impurities in the iron to produce carbon dioxide and other compounds that vented out the top of the furnace as gases. Enough heat was created by these reactions to keep the mixture molten without the use of fuel. Kelly referred to his process as the “air-boiling process”; it was later known as “Kelly’s pneumatic process” because of the role of forced air.
Kelly was encouraged by the results. He succeeded in lessening his dependence on fuel, which had been his main objective. The result, he claimed, was iron that was decarbonized and refined, and equal to the cast iron produced by traditional foundry methods. But Kelly’s firm produced wrought iron, and the product of his experiments lacked the properties he sought.

Western Kentucky a Birthplace of Steel
WKMS (NPR from Murray State University in Murray, Kentucky) produced a news documentary about William Kelly's legacy in May 2015. Listen here.
Improving the Kelly Process
While looking for a way to make high-grade gun metal in 1854, English businessman Henry Bessemer (1813–1898) began an independent series of experiments in which he observed the same phenomenon that Kelly had witnessed—the decarbonzation of molten iron by air alone.
Bessemer secured the British patent for his discovery in 1855 and the U.S. patent in 1856. When Kelly learned that a competitor had received a patent for a process that he had devised years earlier, he appealed to the patent commissioner. The appeal was successful, and Kelly was awarded priority of invention by the U.S. Patent Office, effectively nullifying Bessemer’s U.S. patent.
While each inventor had independently developed a pneumatic process for refining iron, neither yet knew how to control the carbon content of their products. Nor had they demonstrated the ability to apply the process in a factory setting.
Enter Robert F. Mushet (1811–1891), the English metallurgist who would find a way to produce iron with the chemical composition desired. Mushet removed as much carbon as possible from the iron using the pneumatic process, and then he restored the desired amount of carbon by adding a “triple compound” of iron, carbon and manganese, known as spiegeleisen. This resulted in a product with the carbon content iron makers preferred.
Additional improvements, including the introduction of a patented tilting converter by Bessemer, led to a complete commercial process for the manufacture of iron. While chemical and mechanical improvements would continue to be developed by inventors on both sides of the Atlantic, the three key methods for producing commercial quantities of high-quality iron were now known.
It made possible the rapid manufacture of a malleable product of iron, in large quantities and masses, of any carbon content, at a low cost. Its commercial and economic importance cannot be measured.”
—Joseph D. Weeks, American Institute of Mining Engineer presidential address, 1896
Commercial manufacture of iron and steel
For businesses that wished to manufacture iron, a final challenge remained: securing the legal rights to proceed. In Great Britain, Bessemer held the patent for a pneumatic process that could not deliver consistent quality, and Kelly held essentially the same patent in the U.S. Mushet held a patent in both countries for making iron of consistent quality, but his process relied on the patents of Kelly and Bessemer. Meanwhile, Bessemer alone held the patents for the tilting converter.
By 1859, Bessemer’s group in Great Britain gained rights to use each of the three critical processes—pneumatics, the triple compound and his own mechanical designs. Yet in the U.S., where railroad companies produced the greatest demand for iron the world had ever known, business interests continued to jockey for technological control.
In 1866, the U.S. patent owners combined their interests and formed the Pneumatic Steel Association, finally opening the doors to large-scale production in this country. By the 1880s, the U.S. iron and steel industry flourished. It became the world leader in production, generating vast wealth and creating hundreds of thousands of jobs in the process.
Legacy of William Kelly
The story of steel is one of the proud chapters of U.S. industrial history, an advance that propelled the country past its competitors at the dawn of 20th century. The developments of Kelly, Bessemer, Mushet and others fueled the expansion of the manufacture of iron and steel that was a hallmark of the era.
Despite Kelly’s role in demonstrating the first practical development of the pneumatic process for iron manufacturing and Mushet’s ability to produce iron of consistent and desirable qualities, it is Bessemer who is best remembered for establishing the iron industry of the late 1800s. Bessemer assembled the legal protection and business support to commercialize the processes in Great Britain several years before his rivals in the U.S. For his role, Bessemer earned wealth and also fame—the methods that underpinned the entire industry became known as the Bessemer process.
Regardless, Kelly remains an important figure in American steel-making. His discovery that air, blown through molten iron, would both remove impurities and produce heat enough to sustain the molten mass was a key feature of the overall commercial process. Kelly received royalties from his patent, but lived in relative obscurity. He died in Louisville, Kentucky, in 1888.

Landmark dedication and acknowledgments
Landmark dedication
The American Chemical Society will dedicated William Kelly's development of the pneumatic iron process for iron-making as a National Historic Chemical Landmark in ceremonies in Eddyville and Murray, Kentucky, on May 11, 2015. The commemorative plaques at the Lyon County Public Library (Eddyville) and Jesse D. Jones Hall on the campus of Murray State University (Murray) read
In 1847, William Kelly (1811–1888) of Lyon County, Kentucky, began experimenting with a new method for refining iron. His discovery involved blowing air through molten pig iron, a crude form of iron, in a specially-designed cupola furnace. Oxygen in the air combined with carbon in the iron to produce heat without the use of fuel and removed impurities as oxides. Kelly received a U.S. patent for his invention in 1857, establishing priority in the development of this method. When combined with later chemical and mechanical improvements, Kelly’s pneumatic process revolutionized the manufacture of iron and steel, which allowed for the large-scale economical production of steel for use in railroads, skyscrapers, bridges, and other purposes beginning in the late 1800s.
Acknowledgments
Adapted for the internet from “Kelly Pneumatic Iron Process,” produced by the National Historic Chemical Landmarks program of the American Chemical Society in 2015.


Research resources
Further reading
- Kelly Pneumatic Iron Process (National Historic Chemical Landmark booklet; PDF)
- Western Kentucky: A Birthplace of Steel (WKMS news documentary; audio)
Cite this page
American Chemical Society National Historic Chemical Landmarks. Kelly Pneumatic Iron Process. http://www.acs.org/content/acs/en/education/whatischemistry/landmarks/kelly-iron.html (accessed Month Day, Year).


