Plutonium-238 Production for Space Exploration
Dedicated at SRS Heritage Museum in Aiken, South Carolina, on Nov. 1, 2018
Voyages into space are among the most iconic endeavors of the 20th and 21st centuries. Spacecraft that have ventured out to this “final frontier” have relayed stunning images and enlightening scientific data back to Earth, providing knowledge and inspiration to millions of people.
Yet reaching space was a monumental achievement that depended on overcoming numerous difficulties. Even supplying electricity and heat to a spacecraft in flight was a challenge, given the vast distances and intense cold of space. Beginning in the late 1950s, the U.S. met this challenge by developing nuclear batteries known as radioisotope thermoelectric generators (RTGs) and producing plutonium-238 (Pu-238) as their fuel, enabling the exploration of deep space. Together, these two technologies represent an example of the nation’s nuclear and space programs collaborating to develop peaceful uses for radioactive materials.
Contents

After launch, satellites and other spacecraft require continuous current to power guidance and communication systems, to run scientific equipment and to maintain suitable temperatures for operation. Energy systems must be lightweight to facilitate lift-off; robust enough to endure the stresses of launch and the extreme conditions of space; and sufficiently reliable to ensure weeks, months, years and even decades of continuous operation.
Solar power can be used for this purpose in some near-earth missions, but it’s inadequate for missions that venture too far from the sun or that spend considerable time in the shadow of astronomical objects. The relatively short operating life and temperature limitations of chemical batteries, another option, constrain their application in space.
Radioactive materials offer an alternative power source. Such materials, which can be found in nature or produced in a reactor, generate continuous heat through radioactive decay. The rate of decay and type of radiation emitted vary for different radioactive materials, with some decaying rapidly and others more slowly. Finding the right material — one combining good heat generation with the long life required for deep space exploration — was an important goal of researchers and engineers involved in planning space missions.
In 1954, scientists at the U.S. Atomic Energy Commission (AEC) developed the earliest prototype RTG to convert heat emitted by radioactive materials into electrical energy. Critical to their invention was a well-known device called a thermocouple, which utilizes differences in temperature to generate electricity (a process known as the thermoelectric effect). RTGs excel at space applications, because these nuclear batteries are highly stable, lightweight and free of moving parts.
In 1959, a prototype RTG that generated a sustained 2.5 watts of power over 90 days was presented to President Dwight D. Eisenhower as a major advance in the nation’s space program. Supporters of the project saw the need for a more powerful and longer-lasting device for space missions. Fortunately, rapid advances in nuclear chemistry — in the U.S., an outgrowth of the World War II-era Manhattan Project that produced the first nuclear weapons — yielded an array of new radioactive materials. One of these, Pu-238, is nearly ideal for fueling RTGs.

"I firmly believe that nuclear energy provides the most feasible means of accomplishing long voyages in space and many other ambitious missions of our national space program… Because of the exciting panorama of applications, the development of nuclear energy for space is most important. Mankind is only on the verge of the space age. Nuclear power will take us into this age — and close to the planets."—Glenn T. Seaborg, Chairman, U.S. Atomic Energy Commission, June 29, 1962
Different isotopes of a single chemical element are distinguished by the number of neutrons in their nuclei. For example, each atom of the Pu-238 isotope contains 144 neutrons, while each atom of the Pu-239 isotope contains 145 of these neutral particles. Radioactive isotopes are further characterized by their half-life, the time it takes for any given amount of the element to be reduced by half. That happens when the element emits radiation (such as alpha particles) and decays into a lighter element.
Pu-238 has a half-life of 87.7 years, making it a much longer-lasting source of energy than polonium-210, which was used in the 1959 RTG prototype and has a half-life of 138 days. Pu-238 exhibits high heat density and emits primarily alpha particles, which are easily shielded; this makes it safer to handle than most other radioactive materials. High heat density and low shielding requirements both make for a lighter device. Unlike Pu-239, Pu-238 is non-fissile, so it cannot be used in nuclear power plants or nuclear weapons.
The greatest limitation of Pu-238, a manufactured isotope, is the difficulty of making it in sufficient quantities. This task fell to the Savannah River Site (SRS), an AEC facility dedicated to producing, processing and refining nuclear materials.
SRS was established in 1950 near Aiken, South Carolina, primarily to make tritium (an isotope of hydrogen) and Pu-239 for the nation’s nuclear weapons arsenal. The complex included five reactors to produce these radioactive materials and two separations facilities to isolate and purify them.
SRS produced the Pu-239 from a combination of uranium-235 (U-235) and U-238 in the reactors. U-235 in this “fuel” released neutrons that struck U-238, converting some of it into U-239. The U-239 then decayed in a two-step process, first turning into neptunium-239 (Np-239) and subsequently into Pu-239. To limit the build-up of unwanted byproducts, the reaction would be stopped after only a small amount of the uranium was consumed.
Next, the nuclear reaction products were chemically separated by means of the plutonium-uranium extraction (PUREX) process. First, they were dissolved in a solution of nitric acid in water and stirred vigorously with a solution of tributylphosphate (TBP) dissolved in a hydrocarbon solvent. Like oil and water, the two solutions separated when the stirring stopped. The TBP solution captured the plutonium and uranium while the nitric acid/water solution retained unwanted byproducts from the reaction. The TBP solution was removed and treated with additional clean-up steps to purify and separate Pu-239 (for use in weapons) from the uranium (for reuse).
PUREX was pioneered at the University of Chicago as part of the Manhattan Project and improved at various AEC facilities, including SRS. SRS became the first facility to operate the process on an industrial scale when the complex’s separations facility, known as F Canyon, became operational in 1954. A second, H Canyon, went online the following year.
While F Canyon continued to isolate and purify Pu-239 and uranium, SRS scientists modified the H Canyon facility in 1959 to focus on recycling a new type of “enriched” uranium fuel after it was used to produce tritium. The revised method they developed was known as the H-Area Modified, or HM, process. It was similar to PUREX but extracted and purified the partially used enriched uranium from unwanted byproducts. The HM process was also used to separate and purify other valuable nuclear reaction products, including neptunium-237 (Np-237), which formed when U-235 absorbed neutrons and then decayed radioactively. Major C. Thompson (b. 1937), an SRS chemist and solvent extraction expert, led the evolution of the HM process over several decades of operation.
In 1959, the AEC commissioned SRS to convert Np-237 into Pu-238 as fuel for the RTG program. SRS researchers optimized known methods of irradiating an oxide of Np-237 with neutrons to produce Np-238, which decayed into Pu-238 (see front cover). Their first delivery of Pu-238 took place in 1960. Although output was initially small in scale, the team honed the process and expanded facilities, and production grew rapidly in the decades that followed.
Pu-238 produced at SRS was shipped to other AEC facilities and packaged into the fuel used in RTGs until 1978, when the site opened its own RTG fuel fabrication facility.
Through these operations, SRS produced the vast majority of the Pu-238 used in RTGs that powered the nation’s space activities—a total of more than 300 kilograms (approximately 660 pounds) between 1959 and 1988. Only about 10 kilograms (22 pounds) were obtained from sources other than SRS.

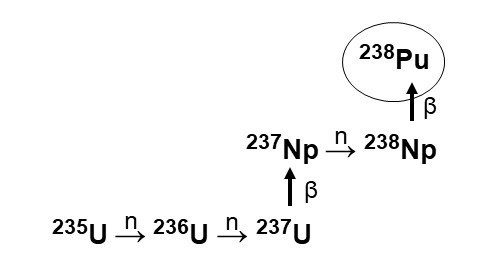
Nuclear reactions show the irradiation of uranium-235 at the Savannah River Site. This produced the intermediate neptunium-237, which was then transformed into plutonium-238.

“Let’s fly this thing. [The Russians are] beating us on other things. Let’s beat them on power.”—President Dwight D. Eisenhower on flying the first radioisotope thermoelectric generator in space in 1961, as reported by George Dix of Martin Company. Spaceflight was the focus of a defensive and technological rivalry between the U.S. and Soviet Union during the Cold War (1945-91).
Pu-238 fueled the first RTG used in space, launched aboard the Navy TRANSIT 4A Navigational Satellite in 1961. Since then, the fuel has been used in nearly every National Aeronautics and Space Administration (NASA) mission that required an RTG, including several Apollo flights (1969–1972), the Viking 1 and 2 Mars landers (1976–1982), Voyager 1 and 2 space probes to the outer planets of our solar system (launched in 1977) and the New Horizons mission to Pluto (launched in 2006)
The Voyager 2 probe—which actually launched before Voyager 1—demonstrates the benefits of a Pu-238-fueled RTG in space missions. It employs three RTGs, each holding 24 Pu-238 oxide fuel packets, which together emitted enough heat to generate a maximum of 470 watts of electrical power at the beginning of the mission. This allowed Voyager 2 to study Jupiter, Saturn, Uranus and Neptune at close range and relay unparalleled scientific data and images back to Earth. Although energy output has fallen over time, the RTG has worked continuously since the mission began on Aug. 20, 1977, and it is expected to continue until at least 2020.
Production of Pu-238 at SRS ended in 1988 as the Cold War came to a close, and other nations also ceased producing the material. Stockpiles of the fuel, used for space missions since then, were projected to be depleted in 2018. The U.S. Department of Energy (successor in 1977 to the AEC) addressed the looming shortage by reestablishing production of Pu-238 at the Oak Ridge National Laboratory for future NASA missions in 2015.
Landmark dedication
The American Chemical Society (ACS) designated the production of plutonium-238 as a National Historic Chemical Landmark (NHCL) in a ceremony at SRS Heritage Museum in Aiken, South Carolina, on November 1, 2018. The commemorative plaque reads:
Space voyages are among the most inspiring and iconic endeavors of the 20th century, yet practical realities have limited spacefaring ambitions. Providing electricity and heat to a spacecraft in flight is challenging, given the vast distances and intense cold of space. Beginning in 1960, the Savannah River Site (SRS) helped meet this challenge by making plutonium-238 to fuel radioisotope thermoelectric generators aboard spacecraft. These nuclear batteries power and warm spacecraft and the research instruments they carry, enabling exploration of deep space. SRS produced nearly all the plutonium-238 for every U.S. mission that has relied on these batteries. Together, the technologies represent a peaceful application of radioactive materials.
Acknowledgements
Written by Keith Lindblom.
The author wishes to thank contributors to and reviewers of this booklet, all of whom helped to improve its content, especially members of the Savannah River National Laboratory (SRNL), the ACS Savannah River Section and the ACS NHCL Subcommittee.
SRNL, the Savannah River Section, the ACS Nuclear Chemistry and Technology Division, and the ACS History of Chemistry Division sponsored the nomination for this Landmark designation.

Research resources
Further reading
- Savannah River Site
- ORNL achieves milestone with plutonium-238 sample
- Plutonium, by World Nuclear Association
- Savannah River Site Museum
- Savannah River National Laboratory
- Voyager mission, by Jet Propulsion Laboratory
- Where does the plutonium come from? by Federation of American Scientists
- What is Uranium? How Does it Work? by World Nuclear Association


