C.V. Raman and the Raman Effect
Designated December 15, 1998, at the Indian Association for the Cultivation of Science in Jadavpur, Calcutta, India.
"I propose this evening to speak to you on a new kind of radiation or light emission from atoms and molecules." With these prophetic words, Professor C. V. Raman of Calcutta University began his lecture to the South Indian Science Association in Bangalore on March 16, 1928. Raman proceeded to describe a discovery that resulted from a deceptively simple experiment. Conducted far from the great centers of scientific research in the Western world, the results would capture the attention of scientists around the world and bring many accolades, including the Nobel Prize, to their discoverer.
Contents
Raman’s Fascination with Light Scattering
Educated entirely in India, C.V. Raman made his first trip to London in 1921, where his reputation in the study of optics and especially acoustics was already known to the English physicists J. J. Thomson and Lord Rutherford, who gave him a warm reception. Raman's specialty had been the study of the vibrations and sounds of stringed instruments such as the violin, the Indian veena and tambura, and two uniquely Indian percussion instruments, the tabla and the mridangam.
But it was the return trip from London to Bombay aboard the SS Narkunda that would change forever the direction of Raman's future. During the fifteen-day voyage, his restless and probing mind became fascinated with the deep blue color of the Mediterranean. Unable to accept Lord Rayleigh's explanation that the color of the sea was just a reflection of the color of the sky, Raman proceeded to outline his thoughts on the matter while still at sea and sent a letter to the editors of the journal Nature when the ship docked in Bombay.
A short time later Raman was able to show conclusively that the color of the sea was the result of the scattering of sunlight by the water molecules. Ironically, it was exactly the same argument that Rayleigh had invoked when explaining the color of the sky — the blue was the result of the scattering of sunlight by the molecules in the air.
Raman was now obsessed with the phenomenon of light scattering. His group in Calcutta began an extensive series of measurements of light scattered primarily by liquids but also by some solids. As a result, Raman was able to explain the blue color observed in the ice of Alpine glaciers.
Raman Measures the Effect of Light Scattering
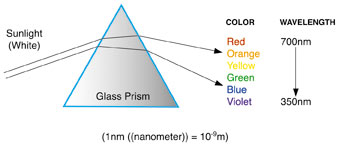
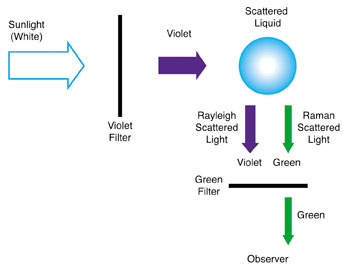
Analysis of light scattered by a liquid is not an easy task, and much of the early work in Calcutta was done by the visual observation of color rather than precise measurements of the light's wavelength as shown in Figure 1 at right. The fundamentals of Raman's crucial experiment are outlined in Figure 2.
The violet light of the solar spectrum is isolated with a violet filter and passed through the liquid sample. Most of the light emerging from the liquid sample is the same color as the incident violet beam: the so-called Rayleigh scattered light. However, Raman and K. S. Krishnan were able to show that some of the scattered light was a different color, which they could isolate by using a green filter placed between the observer and the sample. The advantage of using a visual observation is that several substances can be studied quickly. In his first report to Nature, titled "A New Type of Secondary Radiation," Raman indicated that approximately 60 different liquids had been studied, and all showed the same result — some scattered light had a different color than the incident light. "It is thus," Raman said, "a phenomenon whose universal nature has to be recognized."
The Raman Effect is a very weak effect; only one in a million of the scattered light particles, or photons, actually exhibits the change in wavelength. This explains, in part, why the effect was not discovered earlier. In all of the early light-scattering studies, the excitation source was sunlight, which Raman has described as being plentiful in Calcutta, but it still lacked the desired intensity. The acquisition in 1927 by the IACS of a seven-inch (18 cm) refracting telescope enabled Raman to condense the sunlight and create a more powerful light source for his studies. By early 1928, mercury arc lamps were commercially available, and he switched to this even more intense light source.
Raman knew that visual and qualitative observations alone would not be sufficient information. He methodically set out to measure the exact wavelengths of the incident and Raman scattering by replacing the observer with a pocket spectroscope. He ultimately replaced it with a quartz spectrograph with which he could photograph the spectrum of the scattered light and measure its wavelength. These quantitative results were first published in the Indian Journal of Physics on March 31, 1928.
Raman Effect as the Physicist’s Tool
The significance of the Raman Effect was recognized quickly by other scientists. Professor R. W. Wood of Johns Hopkins cabled Nature to report that he had verified Raman's "brilliant and surprising discovery ... in every particular. It appears to me that this very beautiful discovery which resulted from Raman's long and patient study of the phenomenon of light scattering is one of the most convincing proofs of the quantum theory."
Raman had also recognized that his discovery was important to the debate in physics over the new quantum theory, because an explanation of the new radiation required the use of photons and their change in energy as they interacted with the atoms in a particular molecule. Raman also knew that there was a more important result, remarking in his 1930 Nobel Prize address that "... the character of the scattered radiations enables us to obtain an insight into the ultimate structure of the scattering substance."
In the first seven years after its discovery, the Raman Effect was the subject of more than 700 papers in the scientific literature, mostly by physicists who were using the technique to study the vibration and rotation of molecules and relating those phenomena to the molecular structure. Then, as noted by Raman biographer G. Venkataraman, there was a decline in interest, as "the first bloom of novelty had worn off and physicists were satisfied that they understood the origin of the effect." At the same time, chemists became interested in the Raman Effect as an analytical tool. In James Hibben's words, "The Raman Effect became the adopted child of chemistry."
Raman Effect as a Chemist’s Tool
By the late 1930s the Raman Effect had become the principal method of nondestructive chemical analysis for both organic and inorganic compounds. The unique spectrum of Raman scattered light for any particular substance served as a "fingerprint" that could be used for qualitative analysis, even in a mixture of materials. Further, the intensity of the spectral lines was related to the amount of the substance. Raman spectroscopy could be applied not only to liquids but also to gases and solids. And unlike many other analytical methods, it could be applied easily to the analysis of aqueous solutions. It was a ubiquitous technique, giving information on what and how much was present in a plethora of samples.
The use of Raman spectroscopy as a basic analytical tool changed sharply after World War II. During the war, infrared spectroscopy was enhanced by the development of sensitive detectors and advances in electronics. Infrared measurements quickly became routine operations, while Raman measurements still required skilled operators and darkroom facilities.
Raman spectroscopy could no longer compete with infrared until another development in physics — the laser — revived Raman spectroscopy in a new form beginning in the 1960s.

The Laser and Raman Spectroscopy
Raman understood the need for more intense light sources to amplify the effect and observation of the scattered light. The laser provided an even more intense source of light that not only could serve as a probe exploring the properties of the molecule but could also induce dramatically new effects.
With the development of the Fourier transform (FT) technique and the application of computers for data handling, commercial FT-Raman spectrometers became available in the late 1980s, resulting in resurgence in the use of the original Raman Effect.
The new Raman spectroscopy has been used to monitor manufacturing processes in the petrochemical and pharmaceutical industries. Illegal drugs captured at a crime scene can be analyzed rapidly without breaking the evidence seal on the plastic bag. Chemists can watch paint dry and understand what reactions are occurring as the paint hardens. Using a fiber-optic probe, they can analyze nuclear waste material from a safe distance. Photochemists and photobiologists are using laser Raman techniques to record the spectra of transient chemical species with lifetimes as small as 10-11 seconds. Surface-enhanced Raman spectroscopy is used for studying surfaces and reactions on surfaces. And, according to Kathy Kincade, Raman spectroscopy "has the ability to provide specific biochemical information that may foreshadow the onset of cancer and other life-threatening illnesses."
In his 1928 talk in Bangalore, Raman concluded, "We are obviously only at the fringe of a fascinating new region of experimental research which promises to throw light on diverse problems relating to radiation and wave theory, X-ray optics, atomic and molecular spectra, fluorescence and scattering, thermodynamics, and chemistry. It all remains to be worked out."
Seventy years later scientists are still actively working out the results and practical applications of Raman's deceptively simple experiment.

Biography of Sir C.V. Raman
According to Hindu tradition, Raman was originally named Venkataraman after a Hindu deity, preceded by the initial of his father's first name, Chandrasekhara. In school his name was split to C. Venkata Raman, which later became C.V. Raman. With a father who was a professor of physics and mathematics and a mother who came from a family of Sanskrit scholars, Raman exhibited a precocious nature at an early age. He received a B.A. degree from Presidency College in Madras at the age of 16, placing first in his class and receiving a gold medal in physics.
While studying for his M.A. degree, he published his first research paper in Philosophical Magazine at the age of 18. It was the first research paper ever published from Presidency College.
Because of poor health, he was unable to go to England for further education. With nothing else available in India, in 1907 he passed the Financial Civil Service exam, married, and was posted to Calcutta as assistant accountant general.
Shortly after arriving in Calcutta, Raman began after-hours research at the Indian Association for the Cultivation of Science (IACS). In the first 10 years, working almost alone, he published 27 research papers and led the way for the IACS to become recognized as a vibrant research institute. Much of this early work was on the theory of vibrations as it related to musical instruments. After brief postings in Rangoon and Nagpur, he returned to Calcutta, took up residence next door to the IACS, and constructed a door that led directly into the institute, giving him access at any time. He received research prizes in 1912 and 1913 while he was still a full-time civil servant. He also increased the IACS reputation with his extensive lectures in popular science, holding the audience spellbound with his booming voice, lively demonstrations, superb diction and rich humor.
At the age of 29 he resigned from his lucrative civil service job when Sir Ashutosh Mukherjee, vice-chancellor, Calcutta University, offered him the Palit Chair Professorship. He continued to lecture even though it was not required, and he used the IACS as the research arm of the university. By the time of his first visit to England in 1921, his reputation in physics was well known. Three years later he was elected a Fellow of the Royal Society — only the fourth Indian so honored. That same year he toured the United States, spending four months at the California Institute of Technology through the invitation of Nobel Laureate Robert Millikan.
After discovering the Raman Effect in 1928, he was knighted by the British government in India and received the Nobel Prize in physics in 1930. Three years later, Raman left Calcutta for Bangalore, where he served as head of the Indian Institute of Science. There he continued his work on the Raman Effect and became interested in the structure of crystals, especially diamond. In 1934 he founded the Indian Academy of Science and began the publication of its Proceedings.
In 1948 he became director of the newly constructed Raman Research Institute, where he remained continually active, delivering his last lecture just two weeks before his death. His research interests changed in later years when he primarily investigated the perception of color.
Jagdish Mehra, a biographer, states, "Educated entirely in India, Raman did outstanding work at a time when the small Indian community worked almost entirely in isolation and few made science a career. In fostering Indian science, Raman emerged as one of the heroes of the Indian political and cultural renaissance, along with ... Mahatma Gandhi and Jawaharlal Nehru." But as Raman himself once said, outstanding investigators "are claimed as nationals by one or another of many different countries. Yet in the truest sense they belong to the whole world."
Landmark Designation and Acknowledgments
Landmark Designation
The American Chemical Society and the Indian Association for the Cultivation of Science dedicated The Raman Effect an International Historic Chemical Landmark on December 15, 1998 at the Indian Association for the Cultivation of Science in Jadavpur, Calcutta, India. The plaque commemorating the event reads:
At this institute, Sir C. V. Raman discovered in 1928 that when a beam of coloured light entered a liquid, a fraction of the light scattered by that liquid was of a different color. Raman showed that the nature of this scattered light was dependent on the type of sample present. Other scientists quickly understood the significance of this phenomenon as an analytical and research tool and called it the Raman Effect. This method became even more valuable with the advent of modern computers and lasers. Its current uses range from the non-destructive identification of minerals to the early detection of life-threatening diseases. For his discovery Raman was awarded the Nobel Prize in physics in 1930.
Acknowledgments
Adapted for the internet from "The Raman Effect,” produced by the National Historic Chemical Landmarks program of the American Chemical Society in 1998.
Back to National Historic Chemical Landmarks Main Page.
Learn more: About the Landmarks Program.
Take action: Nominate a Landmark and Contact the NHCL Coordinator.



