Selman Waksman and Antibiotics
Dedicated May 24, 2005, at Rutgers The State University of New Jersey.
Waksman and his students, in their laboratory at Rutgers University, established the first screening protocols to detect antimicrobial agents produced by microorganisms. This deliberate search for chemotherapeutic agents contrasts with the discovery of penicillin, which came through a chance observation by Alexander Fleming, who noted that a mold contaminant on a Petri dish culture had inhibited the growth of a bacterial pathogen. During the 1940s, Waksman and his students isolated more than fifteen antibiotics, the most famous of which was streptomycin, the first effective treatment for tuberculosis.
Contents
- Selman Waksman’s Early Years
- Waksman Moves to America
- Waksman’s Research on Actinomycetes, and the Search for Antibiotics
- The Trials of Streptomycin
- Bringing Streptomycin to Market
- Controversy over the Discovery of Streptomycin
- Selman Waksman’s Later Years
- Research Notes and Further Reading
- Landmark Designation and Acknowledgments
- Cite this Page
Selman Waksman’s Early Life
Selman Waksman called his autobiography My Life with the Microbes. That is also the title of the first chapter of the book, which begins "I have devoted my life to the study of microbes, those infinitesimal forms of life which play such important roles in the life of man, animals, and plants. I have studied their nature, life processes, and their relation to man, helping him and destroying him… I have contemplated the destructive capacities of some microbes and the constructive activities of others. I have tried to find ways and means for discouraging the first and encouraging the second."2
It was a particular kind of microbe found in the soil that intrigued Waksman: the actinomycetes, a group of microorganisms closely related to bacteria. During his long career studying actinomycetes, Waksman realized that many of these microorganisms could inhibit the growth of other microorganisms. That led to the systematic search, starting in the late 1930s, for antimicrobial agents to fight disease, a search made critical by the approach of war.
Selman Abraham Waksman was born and raised in the small town of Novaya-Priluka3 in Ukraine on July 22, 1888 (July 8 according to the old Russian calendar). Waksman described his birthplace as "a bleak town, a mere dot in the boundless steppes." In summer the endless fields produced wheat, rye, barley, and oats. In winter the steppes were blanketed in snow. "The earth was black, giving rise to the very name for that type of soil, tchernozem, or black earth. The soil was highly productive, yielding numerous crops, grown continuously for many years, without diminishing returns."4 While the Waksmans were town dwellers, typical of Jews in the Russian Empire, the fertility of the soil no doubt influenced the young boy's later career choice.
Waksman was named for Solomon, the Kings of Kings, which in Russia had been corrupted over the centuries to Zolmin. His father, Jacob, was a pious man who earned a modest living renting out small houses he owned in neighboring villages. Tending his properties was not a fulltime job; accordingly, he filled his days with prayer and study in the local synagogue. In his autobiography, Waksman described his father's influence upon him as "that of a storyteller" full of tales of wise men who lived in ancient times and of the long history of the Jewish people. Father and son were not close: "He was always in the shadow and did not play that profound part in the life of my boyhood that fathers usually do in the lives of their sons."5
The formative influences on the young Waksman were his mother, Fraida, and her family. His mother was learned, especially for a woman of that period and place. She knew Yiddish literature, had enough knowledge of Hebrew to read scriptures, and could speak Ukrainian. All that served her well, because shortly after she was married, her husband was conscripted into the army. Forced to be independent, she depended on a small dry goods business for income. This thriving business served not only the town of Novaya-Priluka, but also surrounding villages, to which she transported goods on market days. In time, Fraida's mother and sisters came to live with the family, reinforcing the matriarchal environment in which Selman was raised.
Waksman had one sister, Miriam, who died as a young child from diphtheria. She might have survived, but a shipment of antitoxin from Kiev, about two hundred miles away, arrived too late to save her life. Waksman later described how her tragic and unnecessary death influenced him. "As I watched her die," he wrote, "my childish and observant mind may have speculated on the possible effect of the curative agents upon the disease and the potential salvation of her life. Here, for the first time, I was brought in contact with a problem that was later to receive much of my attention."6
At the age of five, Waksman entered the local cheder, or religious school. The education stressed Jewish studies, with the melamed teaching the rudiments of reading scripture and the intricacies of prayer. Within two years, Waksman came under the tutelage of a more advanced teacher who emphasized the prophets and then the Talmud, whose complex interpretations provided the bulk of the intellectual diet. But his mother worried about the limitations of such a parochial education, so she hired private tutors who instructed the ten-year-old boy in Hebrew and Russian as well as literature, history, arithmetic, and geography. While there were holes in his education, Waksman claimed that by the age of thirteen he had a thorough knowledge of biblical and Talmudic writings and of Russian language and literature. In addition to his own studies, Waksman from the age of ten tutored local students, first in the rudiments of reading and writing and later to help prepare the children of the wealthy for entrance into various schools.
As a Jew in the waning days of the Russian Empire, Waksman's access to higher education was limited. He was forced to become what was known as an extern, a student who studied with private tutors and then presented himself at a government-run school to take formal examinations. Upon passing this examination a student received a diploma which conferred all the rights and privileges of regular students. This Waksman successfully did in the larger cities of Zhitomir and Odessa. But following the death of his mother, Waksman decided to forego applying to a university in Russia and instead to follow the example of a number of his relatives and immigrate to the United States. This decision was made easier by the deteriorating status of Jews following the abortive 1905 revolution. The Tsarist government responded to this unrest by employing the age-old tactic of diverting attention from the iniquities of Russian society by whipping up anti-Semitic sentiment among the peasantry. The result was a series of pogroms aimed at Jewish life and property.
Waksman Moves to America
Waksman sailed to Philadelphia in 1910 and quickly departed for Metuchen, New Jersey, where he moved in with a cousin who had a small truck farm which also had a poultry plant. He spent his first few months in America on the farm, becoming familiar with problems of animal nutrition, composting stable manure, and germinating seeds. This appears to have reinforced his interest in the chemical reactions of living bodies, but he had little idea of how to organize such a study. At the suggestion of his cousin, he visited nearby Rutgers College. There he met Dr. Jacob Lipman, a fellow immigrant from Russia, who advised him to abandon an earlier interest in medical school. Instead, Lipman persuaded him that an agricultural curriculum would provide a better training.
He soon enrolled at Rutgers, where he took accelerated course work and spent his fourth year on a research assignment assaying bacteria in culture samples from soil layers. It was while working on this project that Waksman found himself drawn to a particular kind of filamentous bacteria, the actinomycetes. These microorganisms became the focus of his masters' thesis at Rutgers and his doctorate, which he received from the University of California at Berkeley. They were, of course, to be his life's work, although it would be more than two decades before he investigated the possibility of using these microbes to fight other microbes.
In 1916 Waksman became a U.S. citizen and married Deborah Mitnick, affectionately known as Boboli; she came from the same village in Ukraine as he. They would eventually have a son, Byron Halsted Waksman, named after one of his Rutgers' mentors. The first two years of their marriage were spent in Berkeley where Waksman supplemented his graduate fellowship with work for an industrial medical organization, Cutter Laboratories. This relationship established a pattern that was to prove useful in the future but which also led to some embarrassment.
He returned to Rutgers in 1918 as a Lecturer in Soil Microbiology at the college and Microbiologist at the Agricultural Station. He had asked for this title because he was not so much interested in bacteria as in the fungi and actinomycetes among the microorganisms. The broader description of microbiologist would prove to be more apt than the narrower one of bacteriologist as Waksman began his life's work.

"As I watched her die my childish and observant mind may have speculated on the possible effect of the curative agents upon the disease and the potential salvation of her life. Here, for the first time, I was brought in contact with a problem that was later to receive much of my attention."
— Selman Waksman, My Life with the Microbes, (New York: Simon and Schuster
Waksman’s Research on Actinomycetes,
and the Search for Antibiotics
Selman Waksman's enduring fame rests on the discovery of streptomycin, "the culminating point of a painstaking search for antimicrobial agents produced by actinomycetes…"7 These are a group of filamentous microbes, closely related to bacteria in size and physiology but similar to fungi in structure. In fact, they may be considered as an intermediary between bacteria and fungi.
Waksman first became interested in actinomycetes in 1915 as a student at Rutgers. For the next several decades, he studied their occurrence and abundance in soil, their taxonomy, their role in soil processes such as the decomposition of plant and animal residues and the formation of humus, and their relationship to bacteria and fungi. In particular, Waksman and his students calculated the antagonistic effects that acinomycetes had on bacteria and fungi, establishing in the end that perhaps half of all the actinomycetes found in the soil had the capacity to inhibit the growth of other microorganisms.
Early on in the research on the actinomycetes at Martin Hall on the Cook campus at Rutgers, Waksman and his colleagues knew of Streptomyces griseus, the organism that yielded the streptomycin strain, but it would not be tested for its antibiotic producing properties for several decades.8 It was also known that the actinomycetes had toxic effects upon bacteria and fungi.
In 1924, after several years devoted to soil research, Waksman and his wife spent six months in Europe, the first time they had returned since they immigrated to the United States. Part of the trip was sentimental, a return to the town of their birth. This was, of course, the early years of the Russian Revolution when much had been destroyed and little yet rebuilt. Waksman wrote of his impressions: "The greatest misery that one could ever imagine, the greatest catastrophe that the peoples in Russia had ever gone through; the greatest experiment in social and political relations of men can hardly express what we have seen…"9 The Waksmans spent ten days in Priluka, listening to family members and friends describe their plight: "Each one suffered to an extreme. Many cried like children before their father; they came to pour out before us all their sufferings."10
But science was the main reason for the trip. In his autobiography, Waksman referred to the trip as a "grand scientific tour" the purpose of which was to assess his career as a soil microbiologist. "There was no question in my mind," he wrote, "concerning the role of microorganisms in soil processes, but there was a certain question that was continuously arising as to whether I was headed in the right direction."11 To get answers, Waksman visited important laboratories in France, Italy, Germany, and Scandinavia to discuss methods and research with the leading scientists working in the fields of soil biology and chemistry. He returned to the United States stimulated by what he learned and convinced of the need for a comprehensive treatise on soil microbiology, which he published in 1927 as the Principles of Soil Microbiology.
In the 1920s and 1930s, Waksman continued his studies on actinomycetes in the soil, which "resulted in the isolation of numerous new species, development of a system for the generic classification of this group of organisms, and a better understanding of their metabolic processes."12 Over time, he gradually became convinced that these microorganisms could "exert a considerable influence upon the activities of fungi and bacteria in the soil."13 Still, in these years, his major research activity focused on soil microbes and not disease-producing organisms. Two events occurred in 1939 that forced a change in his approach. One was the start of World War II which suggested the need for new agents to control infectious diseases and epidemics certain to arise, especially in tropical theaters. The second event, "a particular stimulus," was the work of RenÉ Dubos, Waksman's former student, who isolated tyrothricin, which destroyed disease-producing bacteria.14
Dubos had shown that it was possible to find bacteria that inhibited the growth of other bacteria. This represented a significant change in the traditional approach to combating infectious disease. The pioneers in the study of infectious diseases, Koch, Lister, and others, had emphasized the necessity of avoiding contamination by soil and other non-sterile matter. Now a researcher was using a soil-derived agent to combat infectious disease. It was logical that Waksman, given the background of his work on soil microorganisms, would be spurred by this conceptual breakthrough to search for agents active against pathogenic bacteria. Unlike Dubos, however, Waksman concentrated on fungi and, especially, actinomycetes.
Waksman approached the search for antibiotics in a novel and systematic way, unlike the chance discovery of penicillin by Fleming, who observed an accidental contamination of a bacterial pathogen by an airborne mold. Waksman and his students screened by looking for growth inhibition zones surrounding single colonies of a series of isolated soil microbes on agar plates growing under a variety of culture conditions. They then proceeded to test the inhibition on specifically targeted pathogenic bacteria. This was painstaking work, as thousands of cultures of different microbes were isolated and then tested for antibacterial activity, but which only a small percentage demonstrated. These were then further tested to discover which would successfully yield microbe-fighting substances in sufficient quantities and then which were not too toxic for therapeutic use. Waksman's screening protocols were to be very successful, yielding around twenty new natural inhibitory agents, with most coming from the actiniomycetes. In fact, it was Waksman who suggested what has become the common term—antibiotics—for these therapeutic agents.15
The first agent isolated in 1940 under the initial screening program was actinomycin by Boyd Woodruff, a Waksman graduate student, who demonstrated that the methodology would yield antibiotic-producing cultures. Actinomycin was active against a broad range of bacteria and even showed promise of attacking a tuberculosis strain, but it proved too toxic for therapeutic use in humans.16 Two years later, Woodruff isolated streptothricin, an antibiotic which exhibited activity against both gram-positive and gram-negative bacteria.17 The researchers were initially excited about streptothricin because, as Waksman said in his Nobel acceptance speech, it "gave promise of filling the gap left by penicillin in the treatment of infectious diseases due to gram-negative bacteria." In addition to being active against gram-positive and gram-negative bacteria, initial tests of streptothricin showed that it was not toxic to animals. However, pharmacology studies demonstrated that streptothricin had a delayed toxic effect in animals and thus could not be used for therapeutic purposes in humans.18
The partial success of streptothricin indicated that Waksman and his students were on the right track. They needed to find a variant that inhibited pathogenic organisms without actually killing host. The breakthrough came in 1943 when Albert Schatz joined the team, and continuing the general research approach of cross streaking pioneered by Woodruff, found two strains of Streptomyces griseus that produced streptomycin. One of these was found by chance when Doris Jones, another Waksman student, tested the tracheal flora of healthy chickens and noted zones of antagonism on several plates.19 The cultures were given to Schatz and from one of them he isolated the active strain of S. griseus, which produced an antibiotic that inhibited both gram-negative and gram-positive bacteria. This was significant since penicillin had no effect on gram-negative bacteria. Even more exciting to the researchers was that streptomycin exhibited activity in vitro activity against Mycobacterium tuberculosis, the Great White Plague.20
The Trials of Streptomycin
Selman Waksman and his team of students knew from their own in vitro tests that streptomycin was active against certain pathogens, especially the bacillus that caused tuberculosis. Waksman also knew that his small laboratory at Rutgers University was not equipped to do further tests, certainly not in vivo ones. Waksman therefore contacted two medical researchers at the Mayo Clinic, William H. Feldman and H. Corwin Hinshaw, to perform animal tests using guinea pigs. Feldman replied on March 7, 1944 that "we are in a position to make such a test" if Waksman could secure sufficient samples of the agent.21
Waksman was able to supply the Mayo Clinic with sufficient samples because of a prior agreement he had reached with Merck & Company in late 1939 under which the giant pharmaceutical company gave Waksman's laboratory a grant for the study of antibiotics. An agreement was drawn up with Merck whereby the company provided chemical assistance, experimental animals for pharmacological evaluation of antibiotics, and large-scale equipment for producing any promising discoveries. In return, Waksman assigned Merck any patents resulting from research in his laboratory. Should any of the patents prove commercially successful, Merck was to pay The Rutgers Foundation a small royalty.22
Armed with samples supplied by Merck, Feldman and Hinshaw began in vivo tests and within two months reported to Waksman that two animals receiving streptomycin "look exceedingly well."23 Throughout 1944, the Mayo Clinic team performed tests on tubercular guinea pigs, tinkering with the dosage to minimize side effects. For example, weight loss in the test animals seemed a problem, but Feldman could report to Waksman in July that "results appear to be satisfactory" when the dosage was reduced.24 In September, when a 60-day in vivo test of a large sample was completed, Feldman wrote that "definite signs of tuberculosis were absent in almost every instance."25 In 1945, clinical trials confirmed the animal results, with Hinshaw reporting in August that thirty-three patients had been treated "and [we] continue to be quite optimistic."26
Bringing Streptomycin to Market
The tests proved that streptomycin was the first effective chemotherapeutic treatment for tuberculosis. It was also effective against a host of other diseases: typhoid fever, cholera, bubonic plague, tularemia, urinary tract infections, and others. As early as 1945, Waksman, realizing that streptomycin would be an important antibiotic, became uncomfortable with the agreement giving Merck exclusive rights to the drug, especially since Rutgers had become the State University of New Jersey the previous year. Waksman believed that prices could be reduced if several companies could manufacture the drug. He also wondered whether Merck alone could meet the demand for streptomycin. Accordingly, Waksman approached Merck requesting abrogation of the 1939 agreement. Merck was "willing to accede to this request," provided a new agreement could be reached.27
Merck was generous: The company agreed to assign the patent rights to Rutgers and to accept a non-exclusive license for the production of streptomycin. Merck also requested, and was granted, a rebate on royalties to compensate the company for money spent in the development of streptomycin. Initially, this agreement appeared to satisfy all parties involved in the development of the antibiotic. Merck was praised for its generosity and Rutgers made licensing agreements with other drug companies.28
Because it attacked a wide spectrum of diseases, including tuberculosis, streptomycin almost immediately began generating huge profits. Originally, The Rutgers Foundation turned over about half the royalties it received from Merck to Waksman. When it became clear that this was a lot of money, Waksman had his share reduced to a fifth. In 1946, when Merck turned over the patents to Rutgers, the foundation was reorganized as The Rutgers Research and Endowment Foundation. By about 1950 Waksman's share was again reduced to ten percent, but since that was still a considerable sum of money, he arranged for half of it to be used to create the Foundation for Microbiology.29
Development and Production of Penicillin
Alexander Fleming: Discoverer of Penicillin and Penicillin Production through Deep-tank Fermentation, were designated National Historic Chemical Landmarks in 1999 and 2008, respectively. Learn more.
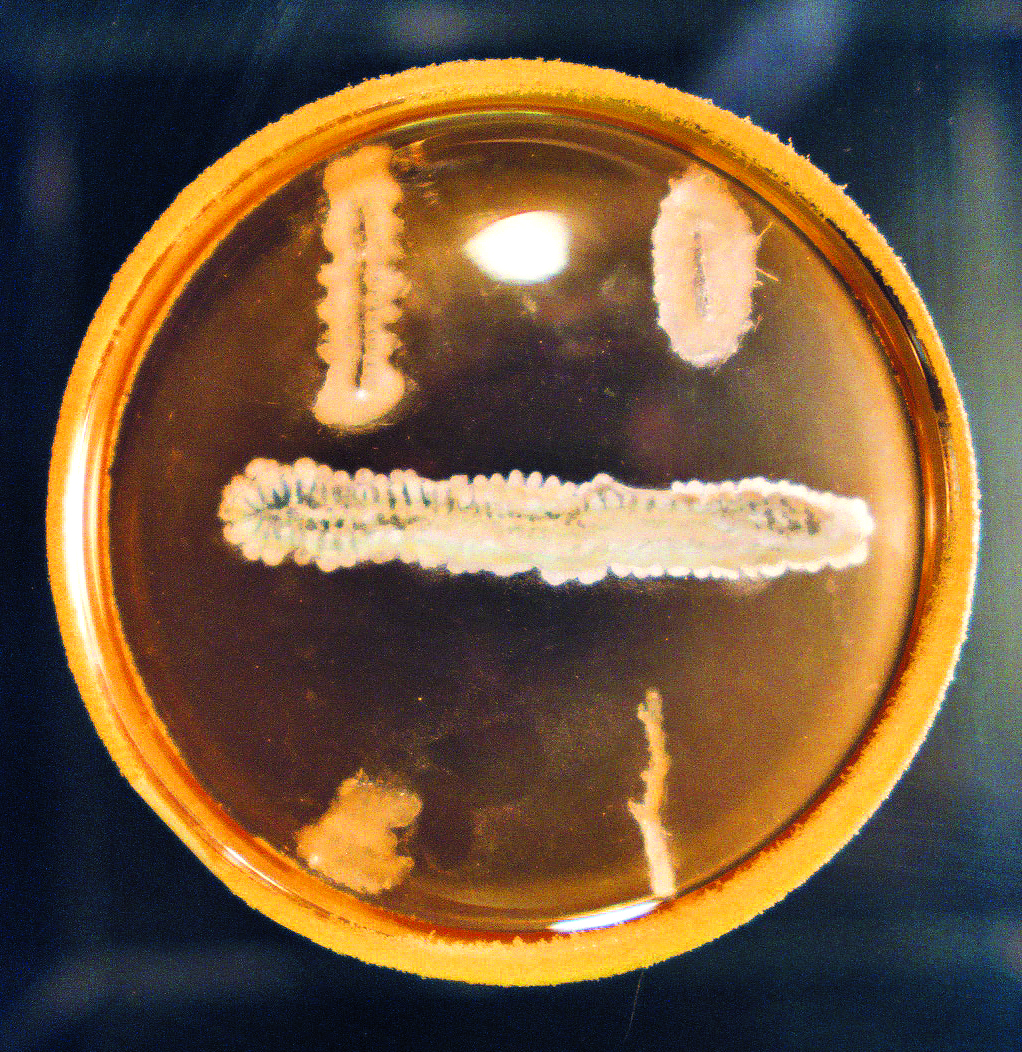
Controversy over the Discovery of Streptomycin
In addition to money, Waksman was being recognized as "the discoverer" of streptomycin. In early 1949, for example, Time ran a story that began: "People are always asking greying [sic] Microbiologist Selman Abraham Waksman, 60, how he discovered the wonder drug streptomycin in 1943."30 Later that year, Waksman's picture graced the cover of Time.31 Awards, plaudits, and recognition from many quarters came Waksman's way.
Given all the money and fame attached to the discovery of streptomycin, it is no surprise that there would be some ruffled feathers. Nonetheless, it came as a shock when Albert Schatz sued Waksman and The Rutgers Research and Endowment Foundation in March 1950. The lawsuit requested Waksman to cease claiming he was the sole discoverer of streptomycin and asked for an accounting of the royalties earned from licenses granted by Waksman and the Foundation. Schatz also sought a substantial amount of the funds received so far.32
Schatz clearly believed he was co-discoverer of the drug as he had performed the basic laboratory work in its isolation and since his name was listed first on the original paper reporting the antibiotic's discovery and second on the patent.33 In addition, his doctoral thesis, which he defended in 1945, was on streptomycin. Initially, however, Schatz appeared content with his public role in the discovery, writing Waksman "to express my appreciation for all that you have done for me both in my undergraduate, graduate and post-graduate work."34 In 1946 a colleague asked Schatz why his name was listed first on the paper announcing streptomycin, and Schatz said that Waksman was "thoughtful and concerned" about his students and did them the honor of putting their names first.35 Nor did money appear to be an issue for Schatz at first, since as late as 1948 he was returning royalty checks to Waksman, writing "I simply would not know what to do with more money if I had it."36 At about the same time Schatz said "I have not the slightest desire for fame, glory, popular acclamation, or a lot of money."37
But just a few months later, Schatz would write Waksman a long letter expressing concern that his contribution to the discovery of streptomycin was being forgotten.38 Waksman replied, in a tone Schatz must have found arrogant and condescending, that "you know very well that you had nothing whatever to do" with the discovery. "Further," Waksman wrote, "you know quite well that we gave you all the credit that any student can ever hope to obtain for the contribution that you have made to the discovery of streptomycin. You know quite well that the methods for the isolation of streptomycin had been worked out in our laboratory completely long before your return from the army, namely for streptothricin. I am sure that your memory does not fail in the fact that the very name streptomycin existed before your return."39 A little later Waksman told Schatz that "you must, therefore, be fully aware of the fact that your own share in the solution of the streptomycin problem was only a small one. You were one of many cogs in a great wheel in the study of antibiotics in this laboratory. There were a large number of graduate students and assistants who helped me in this work; they were my tools, my hands, if you please."40
Clearly Waksman, and others who worked in the laboratory, believed Schatz had made only a minor contribution to the discovery of streptomycin. Schatz was in the laboratory for only three months in 1943; he performed a routine screen according to protocols worked out by Waksman years earlier; and Waksman, based on the success of streptothricin, was clearly looking for an antibiotic with similar properties but less toxicity.41 "Not only did we know the nature of the organism," Waksman wrote in 1950, "but we developed all the methods for its isolation and had the name already available so that it was merely a question of screening a certain number of forms before we had the right organism… It just happened that Schatz was concerned with some of the early isolations and tests, but… Miss Elizabeth Bugie and Miss H. Christine Reilly have made as important contributions, if not more so, in the discovery and development of streptomycin than Schatz has done."42
The scientific community, for the most part, rallied to Waksman's side. Still, there were some misgivings expressed by Waksman supporters over the disposition of royalties. Waksman's colleague at Rutgers, Robert Starkey, told him that "I am sure that your friends have no feeling that you are justified in having any amount of money from the patents that you might wish but they feel hurt that they had been misled with regard to the disposition of the funds."43 Others said that they understood that all money was to go to the Foundation and were "quite surprised that a considerable sum had been diverted to Dr. Waksman. We feel that it is very proper that Dr. Waksman should be rewarded in a material way for his long years of work, but it rather unfortunate in the way the rewarding has worked out."44
In December 1950 the case was settled. The President of Rutgers issued a statement explaining that all parties recognized that Schatz was co-discoverer of streptomycin. Under the agreement Schatz was to receive three percent of the royalties paid to the Foundation, with ten percent going to Waksman and another seven percent split among all who participated in the early work leading to the development of streptomycin. (Waksman later reduced his share to five percent.) Although he agreed to the settlement, Waksman always considered 1950 the "darkest" year in his life.45
Selman Waksman’s Later Years
Streptomycin, of course, was the great success story of the Waksman screening protocols. There would be other antibiotics found, most notably neomycin, isolated by Hubert Lechevalier, which is still in use today as a topical antibacterial agent.46 But it was streptomycin that gained Waksman and his laboratory fame and fortune as well as controversy.
Waksman dedicated part of his personal royalties from antibiotics to create the Foundation for Microbiology in 1951. In addition to and separate from the Foundation, he used another portion of the royalty income to establish the Institute of Microbiology to strengthen the study of the field at Rutgers University. The official dedication of the Institute took place in 1954 with Waksman serving as director for its first four years. The new Institute, a free-standing research facility, had well-equipped laboratories and a fermentation pilot plant.
In 1952 Waksman received the Nobel Prize in Physiology or Medicine for "your ingenious, systematic and successful studies of the soil microbes that have led to the discovery of streptomycin." In his speech at the Nobel dinner, Waksman said:
With the removal of the danger lurking in infectious diseases and epidemics, society can face a better future, can prepare for a time when other diseases not now subject to therapy will be brought under control. Let us hope that in contributing the antibiotics, the microbes will have done their part to make the world a better place to live in.47
The Nobel Prize was one of many awards and plaudits that Waksman received in his later years. He died in 1973.
Research Notes and Further Reading
Research Notes
- Waksman cited this passage, in the quoted translation, at the beginning of the published version of his Nobel Lecture, given on December 12, 1952. "Streptomycin: background, isolation, properties, and utilization," Nobel Lectures, Physiology or Medicine 1942-1962 (Amsterdam: Elsevier Publishing Company, 1964), p. 370.
- Selman Waksman, My Life with the Microbes, (New York: Simon and Schuster, 1954), p.3.
- In his autobiography, Waksman spells the first part of the name of the town as Novaia. However, Novaya is used here as a more accessible transliteration for English speakers. See, Selman A. Waksman, My Life with the Microbes (New York: Simon and Schuster, 1954), p. 17. This biographical section relies heavily on Waksman's reminiscences in his autobiography.
- Ibid.
- Ibid. p. 30.
- Ibid. p. 29.
- "Streptomycin: background, isolation, properties, and utilization," Nobel Lectures, Physiology or Medicine 1942-1962 (Amsterdam: Elsevier Publishing Company, 1964), p. 372.
- Ibid., p. 373.
- Selman A. Waksman, My Life with the Microbes (New York: Simon and Schuster, 1954), p. 145.
- Ibid., p. 146.
- Ibid., p. 120. The title of the chapter on this trip is "Europe Revisited . . . Grand Scientific Tour."
- Selman A. Waksman, "The Background of the Discovery of Streptomycin," typed manuscript, dated 1953? Waksman Papers, Rutgers University, p. 1
- Waksman, My Life, p. 212.
- Waksman, "Background," p. 2.
- Rollin Hotchkiss, "Selman Abraham Waksman," Biographical Memoirs of the National Academy of Sciences 83 (2003): 326; Waksman wrote that it was a name "I suggested in 1941 for 'chemical substances of microbial origin…'" Waksman, "Background," p. 2. See also Selman Waksman, "What is an Antibiotic or Antibiotic Substance," Mycologia Vol. 39, No. 5 (Sept.-Oct. 1947): 565-569.
- Selman Waksman and H. Boyd Woodruff, "Actinomyces Antibioticus, a New Soil Organism Antagonistic to Pathogenic and Non-Pathogenic Bacteria," Journal of Bacteriology, 42 (1941): 231-249. Waksman, "Background," p. 2.
- One of the ways of classifying bacteria is by their color after a particular chemical stain -- the Gram stain -- is applied. Some bacteria stain blue; they are called gram-positive. Other stain pink; these are gram-negative. Gram-positive and gram-negative differ in the kinds of infections they cause. The two types also react differently to antibiotics. In general, gram-negative bacteria are more resistant to antibiotics because their outer membrane prevents drug penetration. See the Merck Manual of Medical Information, Second Home Edition Online. Streptomycin, like streptohricin, attacked both strains, whereas penicillin was ineffective against infections caused by gram-negative bacteria.
- Nobel Lectures, p. 375; Selman Waksman and H. Boyd Woodruff, "Streptohricin, A New Selective Bacteriostatic and Bactericidal Agent, Particularly Effective Against Gram-Negative Bacteria," Proceedings of the Society for Experimental and Biological Medicine 49 (1942): 207-210.
- Waksman believed that the original throat swab came from a sick chicken. He sent his version of the story to George Gray in 1960 for an article Gray was writing for Scientific American. Waksman to Gray, October 5, 1960, a copy supplied to the author by Douglas Eveleigh of Rutgers University. This letter is apparently how the story got into the press and subsequently was picked up by others. See for example, Thomas Dormand, The White Death, (New York: New York University Press, 2000), p. 364.
- Albert Schatz, Elizabeth Bugie, and Selman Waksman, "Streptomycin: A Substance Exhibiting Antibiotic Activity Against Gram-Positive and Gram-Negative Bacteria." Proceedings of the Society for Experimental and Biological Medicine, 55 (1944): 66-69. See Dormandy, The White Death, pp. 360ff and Frank Ryan, The Forgotten Plague: How the Battle against Tuberculosis was Won - and Lost (Boston: Little Brown, 1992), pp. 209ff.
- William Feldman to Selman Waksman, March 7, 1944, Waksman Papers, Rutgers University.
- Selman Waksman, My Life with the Microbes (New York: Simon and Schuster, 1954), pp. 203-204; interview with Boyd Woodruff, conducted by Judah Ginsberg, February 16, 2005. Waksman had long had active dealings with industry. As a graduate student at the University of California, he had worked at Cutter Laboratories; when he returned to Rutgers as a faculty member in 1918, Waksman supplemented his meager salary with a position at Takamine Laboratory in Clifton, New Jersey.
- Feldman to Waksman, May 4, 1944, Waksman Papers.
- Feldman to Waksman, July 31, 1944, ibid.
- Feldman to Waksman, Septemer 19, 1944, ibid.
- Hinshaw to Waksman, August 25, 1945, ibid.
- George Merck to Waksman, February 14, 1945, ibid.
- Hubert Lechevalier, "The Search for Antibiotics at Rutgers University," in John Parascandola, The History of Antibiotics: A Symposium, (Madison, Wisconsin: American Institute of the History of Pharmacy, 1980), p. 118.
- Ibid., p. 119.
- Time, April 4, 1949.
- Ibid., November 7, 1949.
- Lechevalier, "The Search for Antibiotics," p. 116; Waksman, My Life, 289ff; see also Frank Ryan, The Forgotten Plague: How the Battle Against Tuberculosis was Won - and Lost (Boston: Little Brown, 1992), pp.332ff.
- Albert Schatz, Elizabeth Bugie, and Selman Waksman, "Streptomycin: A Substance Exhibiting Antibiotic Activity Against Gram-Positive and Gram-Negative Bacteria." Proceedings of the Society for Experimental and Biological Medicine, 55 (1944): 66-69.
- Schatz to Waksman, May 21, 1949, Waksman Papers.
- Samuel Epstein to Waksman, December 6, 1949, ibid. This letter was written after Schatz first expressed displeasure with his perceived role in the development of streptomycin and the disposition of royalties, but before he filed a lawsuit.
- Schatz to Waksman, November 29, 1948, ibid. On May 3, 1946, Schatz and Waksman transferred the rights for the patent application for streptomycin to the Rutgers Endowment Foundation for one dollar - see the copy of a document in the Waksman papers.
- Schatz to Waksman, January 31, 1948, ibid.
- Schatz to Waksman, Januray 22, 1949, ibid. A year earlier, Schatz wrote Waksman complaining that his career was not going well. January 31, 1948, ibid.
- Waksman to Schatz, January 28, 1949, copy, ibid.
- Waksman to Schatz, February 8, 1949, copy, ibid. In a letter to the lawyer for the Rutgers Foundation, Waksman referred to "the very small part that Schatz has played in the discovery of streptomycin…" Waksman to Russell Watson, copy, December 9, 1949, ibid.
- Boyd Woodruff, in an interview with the author, said Schatz made a chance discovery, "all he had done was a routine screen." Schatz had been in the army but had been discharged early in 1943 for medical reasons.
- Waksman to Donald Reynolds, March 17, 1950, copy, Waksman Papers.
- Starkey to Waksman, May 10, 1950, ibid.
- Reynolds to Starkey, May 26, 1950, copy, ibid.
- Waksman, My Life, p. 285. On the details of the settlement, see Lechevalier, "The Search for Antibiotics," p. 116.
- Selman Waksman and Hubert Lecehevalier, "Neomycin, A New Antibiotic Active against Streptomycin Resistant Bacteria, including Tuberculosis Organisms, Science, 109 (March 25, 1949): 305-307.
- Göran Liljestrand, ed., Les Priz Nobel en 1952 (Stockholm: Nobel Foundation, 1953).
Further Reading
- Selman Waksman’s Antibiotics Work Recognized by the ACS (Rutgers, The State University of New Jersey)
- Waksman Institute of Microbiology History (Rutgers, The State University of New Jersey)
- Selman Waksman Biography (Nobelprize.org)

Landmark Designation and Acknowledgments
Landmark Designation
The American Chemical Society designated Selman Waksman’s isolation and development of antibiotics as a National Historic Chemical Landmark in a ceremony on May 24, 2005, at Rutgers, The State University of New Jersey, at Martin Hall on the Cook Campus and the Waksman Institute on the Busch Campus. The text of the plaque commemorating the landmark reads:
Here, in Martin Hall, Selman A. Waksman and his students isolated antibiotics produced by actinomycetes, most notably streptomycin, the first effective pharmaceutical treatment for tuberculosis, cholera, and typhoid fever. They also isolated neomycin, used as a topical antibacterial agent. These discoveries emerged from Waksman's research program, which developed novel screening protocols for detecting antimicrobial agents in the soil. Waksman received a Nobel Prize in 1952 for "ingenious, systematic and successful studies of the soil microbes" that led to the discovery of streptomycin.
Acknowledgments
Adapted for the internet from “Selman Waksman and Antibiotics,” produced by the National Historic Chemical Landmarks program of the American Chemical Society in 2005.
Back to National Historic Chemical Landmarks Main Page.
Learn more: About the Landmarks Program.
Take action: Nominate a Landmark and Contact the NHCL Coordinator.



