By: Kevin R. Campos1 and Guy Humphrey1
1Process Research & Development, Merck & Co., Inc., Kenilworth, NJ, USA


Introduction:
In the past two decades, a surge of interest in Green Chemistry has driven chemists to minimize environmental impacts in developing synthetic methods. Despite these efforts, the pharmaceutical industry generates the most waste per kg of product manufactured, when comparing Process Mass Intensity (total mass of materials required to produce 1 kg of product) across all chemical manufacturing sectors. This outcome can be attributed to the unique synthetic requirements for each new chemical entity or the necessity of lengthy, multi-step operations to convert commodity chemicals, chemicals manufactured and readily available in bulk from multiple suppliers that serve as primary building blocks for specialty chemical and pharmaceutical intermediates, to the desired active pharmaceutical ingredient (API). We, however, argue that this failure is, instead, an opportunity for the pharmaceutical industry to elevate expectations on manufacturing processes, inspiring greater innovation in synthetic route design and inventing creative solutions to develop green and sustainable processes.
In September 2015, the United Nations General Assembly established 17 Sustainable Development Goals (SDGs), many of which align with our company’s mission, to translate fundamental breakthroughs into meaningful medicines and vaccines that save and improve lives globally. We believe that it is our social obligation to not only invent medicines for the benefit of patients, but also to manufacture these important new therapies while protecting and preserving the communities, in which we live and work.
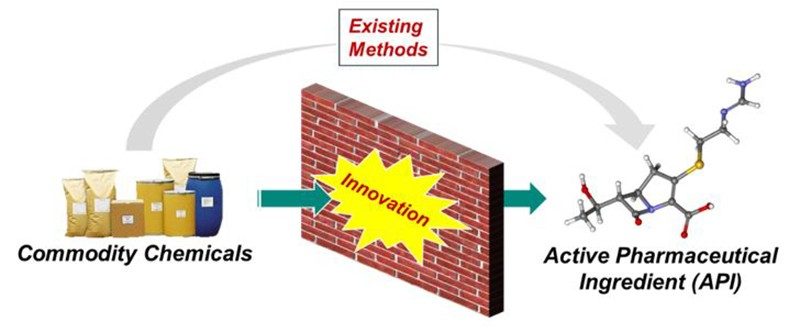
In Process Research and Development, when we embark on the development of a commercial manufacturing process for a therapeutic candidate, we strive to design the most direct method to convert commodity chemicals into the desired molecule with zero waste (Figure 1). Inevitably, many of the transformations that we imagine do not exist, and we must apply creative thinking to strategically invent novel synthetic methods to achieve our aspirational goal. Our company’s investment in a mindset of innovation provides countless benefits to how our medicines are manufactured, minimizing the environmental impact of our processes, maximizing the access of our medicines to patients worldwide, and influencing the field of synthetic chemistry as we do it.
Figure 1. Process R&D’s strategy for commercial manufacturing process definition
Getting off to a good start
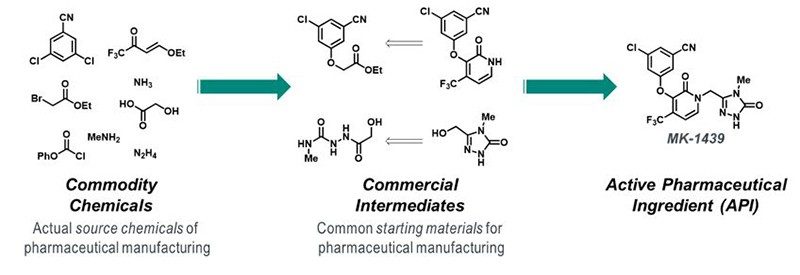
The sustainability potential of any manufacturing process is inherent to the route design: attempts to improve the sustainability of a poorly designed synthetic route will only lead to incremental improvements. Retrosynthetic logic and forward planning are foundational to achieving sustainability goals, but, too often, the failure to define appropriate source chemicals dilutes the impact of innovations later in the synthesis. Chemists come to rely on the multitude of starting materials that are available commercially at large scale, many at modest cost, forgetting that the vast majority of these require chemical synthesis. In fact, the majority of commercial intermediates used in API syntheses originate in Asia, and recent actions by the Chinese Ministry of Ecology and Environment agency have resulted in regulatory action against 40% of the factories across 30 provinces, raising definitive supply chain concerns in the global pharmaceutical industry. Often the chemistry used to prepare such starting materials fails to align with sustainability principles, and, therefore, the overall negative environmental, health and safety impacts of pharmaceutical manufacture are unknown. Compounding this gap is a reluctance of the pharmaceutical industry to incentivize suppliers to adopt green and sustainable chemistry or penalize those that do not meet the environmental sustainability bar. Our commitment to develop the most efficient and sustainable process, starting from true commodity chemicals, reduces future supply chain risk, lowers cost and, most importantly, places ownership and responsibility for all the chemistry used to make the API with the marketing authorization holder (MAH).
Figure 2. Example of API retrosynthesis to commodity chemicals
Chemistry and technology innovation
In order to increase the efficiency of manufacturing routes to achieve our desired aspirational levels, the selection of reactions and technology available to and utilized in industrial laboratories, as well as at CROs, needs to increase significantly. Early adoption and translation of fundamental advances in organic synthesis by pharmaceutical scientists is critical to innovation in green and sustainable commercial process design. Strategic investments in new technology are also required in order to accelerate the discovery efforts towards transformative chemical processes. For example, application of high-throughput experimentation (HTE) reaction discovery tools allows hundreds of milligram-scale experiments to be run simultaneously, serving to accelerate new reaction discovery, mechanistic understanding and reaction optimization. Additionally, the use of computational and predictive science aids in mechanistic understanding and enables the discovery of highly selective catalysts designed to promote novel transformations. In many cases, these are organocatalysts designed to be stable and recyclable, often obviating the use of unsustainable rare-earth metal catalysts. Photoredox catalysis, including nickel and copper metallaphotoredox, has emerged as a powerful technology, enabling novel transformations and innovative chemistry solutions to be discovered and implemented, using an inexpensive and non-hazardous energy source. Academic-industrial partnerships are an essential part of the technology and transformation innovation process. Industry engagement of academic scientists in the fundamental science needed for ground-breaking advancement in sustainable science, while maintaining protection of intellectual property, is clearly feasible given that novel transformations rarely require exact chemical structures to initiate discussion.
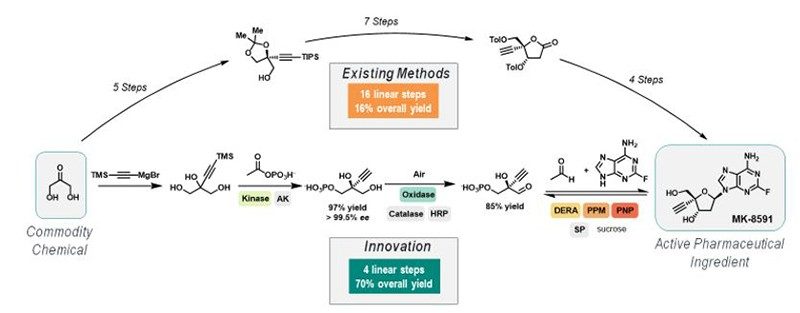
As acknowledged in the 2018 Nobel Prize for Chemistry, biocatalysis (the utilization of enzymes as catalysts), provides a platform for seemingly unlimited chemistry innovation with unparalleled sustainability potential. An example that highlights the true potential of biocatalysis was the recent demonstration of a nine-enzyme biocatalytic cascade to synthesize the investigational HIV nucleoside reverse transcriptase translocation inhibitor, islatravir, in only four chemical steps, from commodity chemicals. This process has a significantly reduced lead time, a 4x improved yield, and nearly 10x reduction in the process mass index (PMI) relative to previous highly optimized route, which is accomplished in 14-16 steps using existing method. This example highlights the massive benefit of a strategy that relies on innovative solutions versus existing chemical methods (Figure 3). The application of engineered enzymes to carry out novel, exquisitely selective and inherently sustainable chemistry is exciting and will undoubtedly play a significant role in how the pharmaceutical industry addresses sustainability opportunities.
Figure 3. Process R&D’s strategy in action: Islatravir commercial process
Process Optimization
Establishing true commodity source chemicals and identifying the most direct route and discovery of enabling transformations are essential elements to designing the most direct method to access the active pharmaceutical ingredient; however, the transformation of a synthetic route to the most sustainable process for commercial production requires strategic thinking and a commitment to the twelve principles of green chemistry, developed by Paul Anastas and John Warner, with an aspirational goal of a zero-waste process. Of utmost importance is solvent consolidation and reduction, as solvent waste is a major contributor to the overall waste and carbon footprint for pharmaceutical manufacturing. Wherever possible, chemical reactions are telescoped or direct isolations are implemented to avoid aqueous work-ups and solvent switches which are both laborious and waste intensive. In addition to reducing solvent usage, it is important to reduce the number of solvents used in a process to aid in solvent recycling/reuse. In many cases, through deep understanding of reaction mechanism, a suitable solvent can be identified for sequences of reactions. In some instances, continuous processing can be implemented to improve the performance, productivity, safety and robustness of commercial manufacturing. Once the commercial process parameters are optimized for sustainability, there are additional opportunities to minimize waste through solvent, reagent and catalyst recycling. We aspire to create manufacturing processes in which every atom of every reagent is either incorporated into the active pharmaceutical ingredient or recycled. To measure progress and drive continuous improvement, we employ metrics such as Process Mass Intensity (PMI) and Life-Cycle Assessment (LCA) to compare our commercial manufacturing processes to prior or alternative synthetic processes, providing a measure of the effectiveness of our strategy, several of which are shown in Figure 4. As a founding member of the ACS Green Chemistry Institute Pharmaceutical Roundtable, we have worked with our industry peers to develop simple tools - available to the general public - to measure and compare process performance from a sustainability perspective.
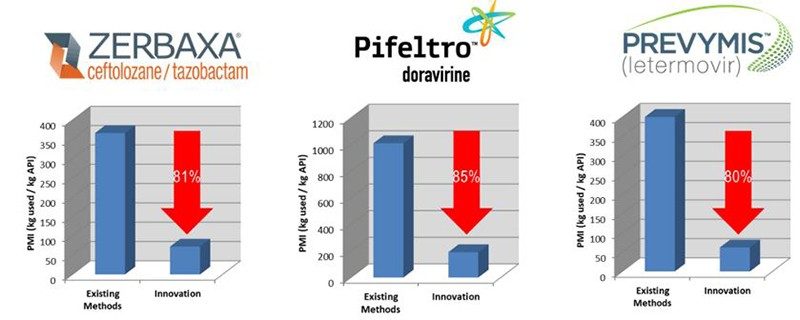
Figure 4. The Impact of Innovation on PMI reduction for recent products launched by our company
Conclusion:
In 2020, the COVID-19 pandemic presents an urgent health crisis, the magnitude of which we have not experienced in over a century. With nearly 25 million people infected, and over 800,000 deaths as a result of SARS COV-2 infection, the scale at which therapies such as Remdesivir or the investigational therapy MK-4482, will need to be manufactured for the world’s population is staggering. To maximize our benefit to human health, we need to elevate our expectations for commercial manufacturing, not just delivering these life-saving medicines to patients in need, but also manufacturing them in a way that minimizes the environmental impact on our global communities. To accomplish this aspirational goal, we must: (1) imagine the most direct way to convert inexpensive commodity feedstocks into the target molecule; (2) invent new synthetic methods to transform those ideas into reality; (3) develop that synthetic route into a highly efficient and sustainable process that can be run in any commercial facility on the planet. According to the US Environmental Protection Agency, the Green Chemistry Challenge Awards “recognize chemical technologies that incorporate the principles of green chemistry into chemical design, manufacture, and use.” We aspire to a day when every commercial product launched by the pharmaceutical industry is a strong contender for this prestigious award.
This article has been edited for length and clarity. The opinions expressed in this article are the author's own and do not necessarily reflect the view of their employer or the American Chemical Society.
Copyright 2022 American Chemical Society (All Rights Reserved)