What molecule am I?
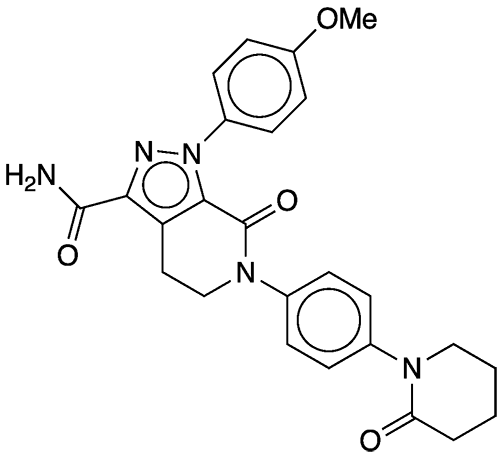
Apixaban1 is an anticoagulant drug that was first described in several mid-2000s patent applications. In one such filing, Rafael Shapiro and co-inventors at Bristol-Myers Squibb (BMS, New York City) disclose processes for preparing 4,5-dihydro-pyrazolo[3,4-c]pyrid-2-ones, of which apixaban is an example. A patent, US 7,396,932, was awarded in 2008.
BMS and Pfizer (New York) codeveloped apixaban under the brand name Eliquis. The drug was approved for use by the European Medicines Agency in 2011 and by the US Food and Drug Administration in 2012.
Apixaban is used to treat or prevent blood clots, including deep vein thrombosis, which can lead to pulmonary embolism. It can also prevent stroke in patients with nonvalvular atrial fibrillation. It is often given to patients after hip or knee replacement surgery to prevent clotting during recovery. It is administered orally and is safer and more convenient than warfarin, the medication previously used for these purposes.
Apixaban’s mode of action is factor Xa inhibition. Factor Xa is activated by the hydrolysis of factor X, an enzyme that is a component of the coagulation (clotting) cascade. Factor Xa, in turn, catalyzes the conversion of prothrombin to thrombin, the enzyme that promotes the formation of fibrin clots.
1. 1H-Pyrazolo[3,4-c]pyridine-3-carboxamide, 4,5,6,7-tetrahydro-1-(4-methoxyphenyl)-7-oxo-6-[4-(2-oxo-1-piperidinyl)phenyl]-.
Apixaban hazard information*
| Hazard class** | GHS code and hazard statement | |
|---|---|---|
| Skin corrosion/irritation, category 2 | H315—Causes skin irritation | |
| Serious eye damage/eye irritation, category 2A | H319—Causes serious eye irritation | |
| Specific target organ toxicity, single exposure, respiratory tract irritation, category 3 | H335—May cause respiratory irritation | |
| Specific target organ toxicity, repeated exposure, category 1 | H372—Causes damage to organs through prolonged or repeated exposure | |
*Compilation of multiple safety data sheets.
**Globally Harmonized System (GHS) of Classification and Labeling of Chemicals. Explanation of pictograms
Molecules from the journals
Nickel molybdate1 (NiMoO4) and nickel tungstate2 (NiWO4) are salts that were originally used as catalysts for organic reactions. In the early 1950s, J. Rooley, C. S. Rohrer, and O. W. Brown* at Indiana University (Bloomington) found that both compounds, after reduction with hydrogen, catalyze the conversion of 1-nitropropane to propylamine in yields approaching 95%.
Recently, the two salts have been used in the electrocatalysis of the oxygen evolution reaction from water; but the active form of the catalysts had not been established. This July, Anubha Rajput, Mrinal Kanti Adak, and Biswarup Chakraborty* at the Indian Institute of Technology (Delhi) reported that when nanoparticles of the compounds are deposited on nickel anodes, NiWO4 remains the catalytic species; but NiMoO4 electrolytically converts to NiO(OH) to become the active catalyst.
Mesodiphenylhelianthrene3 (MDH) is a polycyclic aromatic hydrocarbon derivative that was first mentioned in the chemical literature in 1947 by French chemists Charles Dufraisse and Georges Sauvage*. They synthesized the deep-violet MDH from helianthrone4 and observed that it readily reacts with atmospheric oxygen to produce yellow-colored mesodiphenylhelianthrene endoperoxide5 (MDHPO). When heated to 180° C, the peroxide cleanly loses oxygen to regenerate MDH.
In 1983, S. Hubig and co-workers at the Institute for Physical and Theoretical Chemistry (Frankfurt, Germany) first described MDH as a chemical actinometer, a substance used to quantify the amount of irradiation that produces a chemical reaction. They recommended MDH as “a convenient, reliable, and wavelength-independent actinometer for the visible wavelength range.”
Fast-forward to July 2022: Bernhard Spingler and colleagues at the University of Zurich synthesized MDH in five steps from commercially available 1-aminoanthraquinone. They then determined the crystal structures of MDH, intermediates isolated during its synthesis, and finally MDHPO. They confirmed their results with theoretical calculations.
1. CAS Reg. No. 14177-55-0.
2. CAS Reg. No. 14177-51-6.
3. CAS Reg. No. 86950-18-7.
4. CAS Reg. No. 475-63-8.
5. CAS Reg. No. 86950-19-8.
Molecules from the Journals
MOTW briefly describes noteworthy molecules that appeared in recent ACS journal articles. See this week's
edition below.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
Apixaban fast facts
| CAS Reg. No. | 503612-47-3 |
| Empirical formula | C25H25N5O4 |
| Molar mass | 459.50 g/mol |
| Appearance | White to pale yellow crystals or powder |
| Melting point | 235–240 °C |
| Water solubility | Uncertaina |
a. Reported values range from 0.04 to 110 mg/L.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

