What molecule am I?
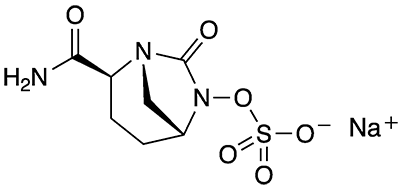
Avibactam sodium and its free acid, avibactam, are diazabicyclooctane (DBO)–based, non-β-lactam β-lactamase inhibitors (BLIs) that are used to treat multi-drug–resistant Gram-negative bacterial pathogens. The compounds were jointly developed by Actavis Generics (now part of Teva Pharmaceutical Industries) and AstraZeneca.
In 2015, avibactam sodium, in combination with ceftazidime, a cephalosporin antibiotic, was approved by the US Food and Drug Administration. The dual-drug’s target was complex urinary tract and intra-abdominal infections caused by Gram-negative, often hospital-acquired, bacteria.
Last year, in the continuing quest to combat multi-drug–resistant bacteria, Eric M. Gordon, Matthew A. J. Duncton, and Mark A. Gallop at Arixa Pharmaceuticals (Palo Alto, CA) prepared analogues of avibactam that are more orally available than the free acid or its salt. (Currently, avibactam can be administered only intravenously; the only orally available BLI on the market is the less useful clavulanic acid.)
The researchers prepared prodrugs of avibactam in which various moieties form esters of the sulfate group. (Historically, charged sulfate groups on drug molecules were seen as strong obstacles to oral bioavailability.) The derivatives with the highest oral availabilities to laboratory animals had a quaternary carbon atom in the β position of the added sulfate ester and a carboxylate ester as one of the substituents on the quaternary carbon (i.e., –OSO3–CH2–CR1R2–CO2R3).
The authors believe that their avibactam analogues are orally available because of steric hindrance to hydrolysis provided by the sulfate ester and the gem-disubstituent/trimethyl lock effect that brings the carboxylate nucleophile closer to the eventual displacement site. In a simulation of in vivo conditions, they demonstrated that the prodrug can be cleaved under mild conditions to release the unesterified avibactam.
Avibactam sodium hazard information
| GHS classification*: skin irritation, category 2 | |
| H315—Causes skin irritation | |
| GHS classification: skin sensitization, category 1 | |
| H317—May cause an allergic skin reaction | |
| GHS classification: serious eye damage, category 1 | |
| H318—Causes serious eye damage | |
| GHS classification: respiratory sensitization, category 1 | |
| H334—May cause allergy or asthma symptoms or breathing difficulties if inhaled | |
| GHS classification: specific target organ toxicity, single exposure, respiratory tract irritation, category 3 | |
| H335—May cause respiratory irritation | |
| GHS classification: germ cell mutagenicity, category 2 | |
| H341—Suspected of causing genetic defects | |
| GHS classification: reproductive toxicity, category 2 | |
| H361—Suspected of damaging fertility or the unborn child | |
| GHS classification: specific target organ toxicity, single exposure, category 1 | |
| H370—Causes damage to organs | |
| GHS classification: hazardous to the aquatic environment, long-term hazard, category 4 | |
| H413—May cause long-lasting harmful effects to aquatic life | |
*Globally Harmonized System of Classification and Labeling of Chemicals. Explanation of pictograms.. Note: Most safety data sheets list fewer, and in some cases, different, hazard statements.
Avibactam sodium fast facts
| CAS Reg. No. | 1192491-61-4 |
| Empirical formula | C7H10N3NaO6S |
| Molar mass | 287.22 g/mol |
| Appearance | White crystalline powder |
| Melting point | Not reported |
| Water solubility | ≈10 g/L |
MOTW update
Oxytocin was the Molecule of the Week for February 20, 2012. It is a pituitary hormone that contracts the uterus during labor and stimulates lactation in nursing mothers, among other functions. Now, Haruhito Higashida at Kanazawa University (Japan), Satoshi Shito at Hokkaido University (Sapporo, Japan), and colleagues are exploring oxytocin derivatives to develop longer lasting, more potent drugs to combat autism and depression.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

