What molecule am I?
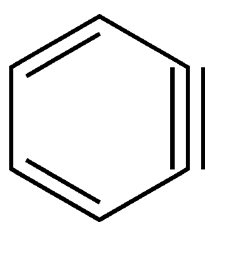
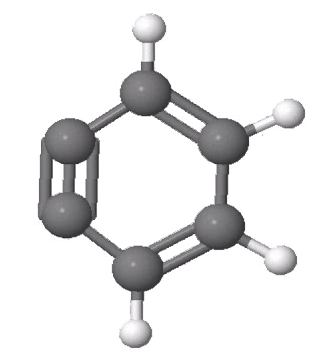
Benzyne is a highly reactive organic molecule that has not been isolated. Its existence only can be inferred by the stable molecules it produces. Its instability is the result of its extremely strained triple bond.
One of the first demonstrations of the existence of benzyne was reported in 1940 by Georg Wittig1 and colleagues at the University of Freiburg (Germany). They combined fluorobenzene and phenyllithium at –78 ºC to produce o-fluorophenyllithium, which, when warmed to room temperature, eliminated lithium fluoride. The resulting high-energy cycloalkyne reacted with another phenyllithium molecule to form o-biphenyllithium.
In 1953, carbon-14 labeling experiments conducted by John D. Roberts2 and co-workers at MIT (Cambridge, MA) showed that the transitory triple bond in benzyne is electrically neutral—as opposed to the double-bond zwitterion that Wittig proposed. In one example, the reaction of [1-14C]chlorobenzene with ammonia gives equal amounts of [1-14C]aniline and [2-14C]aniline, establishing the equivalency of the adjacent carbon atoms.
For more on the history and utility of benzyne, see “The Chemistry of Benzyne” by Joseph F. Bunnett at Brown University (Providence, RI) and “The Benzyne Story” by Curt Wentrup at the University of Queensland (Brisbane, Australia).
1. Wittig won the 1979 Nobel Prize in Chemistry for developing the eponymous olefin synthesis in which an aldehyde or ketone is treated with an organohalide-derived triphenylphosphonium ylide.
2. Roberts, despite his pioneering work in physical and organic chemistry, including the development of nuclear magnetic resonance spectroscopy as an important analytical tool, never won the Nobel Prize.
MOTW update: August 17, 2020
The article above began, “Benzyne is a highly reactive organic molecule that has not been isolated.” A sharp-eyed reader wrote to say that this isn’t exactly true. In 1997, Ralf Warmuth at UCLA generated benzyne in the inner cavity of a hemicarcerand by irradiating incarcerated benzocyclobutenedione at –196 ºC, followed by irradiating the resulting hemicarcerand–benzocyclopropenone under the same conditions.
Benzyne fast facts
| CAS Reg. No. | 462-80-6 |
| SciFinder nomenclature | 1,3-Cyclohexadien-5-yne |
| Empirical formula | C6H4 |
| Molar mass | 76.10 g/mol |
MOTW update:
August 10, 2020
Thimerosal was the Molecule of the Week for August 8, 2011. It is a mercury-based topical antiseptic and antifungal agent that is sold under the trade name Merthiolate. It is also used as a vaccine preservative. Timerosal is claimed to be an inert ingredient in medications, but a recent study performed at the University of California, San Francisco, and the Novartis Institutes for BioMedical Research showed that it reaches blood levels in rats that may interact with biological targets. This finding doesn’t necessarily imply toxicity but it might be sufficient to prompt drug manufacturers to revisit vaccine formulations.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

