What molecule am I?
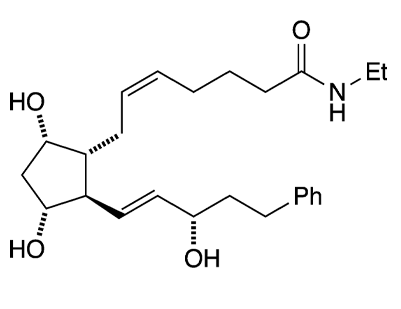
Bimatoprost is an almost 20-year-old drug that has medical and cosmetic uses, both pertaining to the eyes. It is closely related to the prostaglandin family of hormones.
In 2001, the US Food and Drug Administration approved a new drug application from Allergan (Irvine, CA) for using bimatoprost eyedrops to lower intraocular pressure in patients suffering from open-angle glaucoma. Seven years later, FDA approved Allergan’s application for a cosmetic use of bimatoprost: increasing the length, thickness, and darkness of eyelashes. Just last year, FDA accepted Allergan’s application for a sustained-release bimatoprost implant that lets glaucoma patients avoid the need for eyedrops.
Bimatoprost has several adverse side effects. The most frequent one is conjunctivitis, which occurs in more than 10% of patients. Others include blurred vision, redness, burning, and darkening of the iris. Eyelid infection and darkening can occur when the drug is used on eyelashes.
In 2015, Diego Romano and co-workers at the University of Milan (Italy) reported an improved synthesis of bimatoprost and its cousin latanoprost that uses enzymatic reactions. Then, in 2019, bimatoprost was a “guinea pig” during the development of a synthesis-planning program that combines human knowledge with machine learning.
In 2012, Bartosz A. Grzybowski of the Ulsan National Institute of Science and Technology (South Korea) and the Polish Academy of Sciences (Warsaw) published Chematica, a program that uses chemical information to generate synthetic sequences. Grzybowski and colleagues updated Chematica by adding machine-learning algorithms that use the software’s database and chemistry experts’ insights to optimize the practicality of the reaction sequences it produces.
Significantly, the hybrid version of Chematica can identify seldom-used reactions to generate sequences that the original version was unable to find. Bimatoprost synthesis is an example of the use of reactions that did not show up before the original Chematica program was “trained”.
Bimatoprost hazard information
| GHS* Hazard class | Hazard statement |
|---|---|
| Not a hazardous substance or mixture |
*Globally Harmonized System of Classification and Labeling of Chemicals.
Bimatoprost fast facts
| CAS Reg. No. | 155206-00-1 |
| SciFinder nomenclature | 5-Heptenamide, 7-{(1R,2R,3R,5S)-3,5-dihydroxy-2-[(1E,3S)-3-hydroxy-5-phenyl-1-penten-1-yl]cyclopentyl}-N-ethyl-, (5Z)- |
| Empirical formula | C25H37NO4 |
| Molar mass | 415.57 g/mol |
| Appearance | White powder |
| Melting point | 67–68 ºC |
| Water solubility | 19 mg/L (est.) |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

