What molecule am I?

Fifteen years ago, Andre Geim and Konstantin Novoselov at the University of Manchester (UK) prepared and characterized graphene, the fourth allotrope of elemental carbon after diamond, graphite, and amorphous carbon. For their work, the researchers received the 2010 Nobel Prize in Physics.
Now it’s boron’s turn. In 2015, an international team of scientists led by Nathan P. Guisinger at Argonne National Laboratory (IL); Mark C. Hersham at Northwestern University (Evanston, IL); and Artem R. Oganov1 at Stony Brook University (NY), the Skolkovo Institute of Science and Technology (Moscow), and the Moscow Institute of Physics and Technology synthesized borophene, a new allotrope of elemental boron.
Graphene and borophene are similar in that they consist of single sheets of their respective elements. For their mass, both are extremely strong structurally; and they exhibit great flexibility without breaking. But there are important differences: Graphite sheets are planar and have a hexagonal lattice (“chicken-wire”) structure. In contrast, borophene sheets are slightly curved and so far have been found to exist in three suballotropes with differing arrangements of triangles and hexagons.
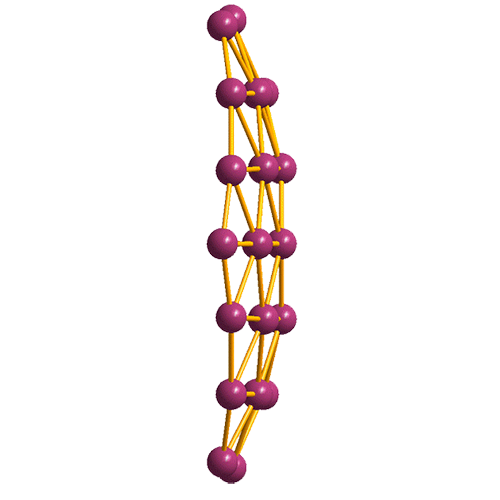
In 2014, before borophene was discovered, Jun Li, Lai-Sheng Wang, and colleagues at Brown University (Providence, RI) and Tsinghua University (Beijing) prepared B36, a quasiplanar layer of boron atoms that consists of triangular structures surrounding a hexagonal “hole” (see images). The authors stated that this arrangement “can be viewed as a potential basis for extended two-dimensional boron sheets.” As predicted, borophene was “born” the following year.
In March of this year, Jin-Cheng Zheng and coauthors at Xiamen University (China), the National University of Singapore, and Xiamen University Malaysia (Selangor) wrote an excellent review of borophene and its myriad potential uses.
1. Oganov is an outstanding young chemist, physicist, and materials scientist. For some of his earlier work, see Molecule of the Week for May 1, 2017.
Borophene fast facts
| CAS Reg. No. | 1623004-11-4 |
| Empirical formula | Bn |
| Molar mass | 10.81n g/mol |
B36 fast facts
| CAS Reg. No. | 390395-11-6 |
| Empirical formula | B36 |
| Molar mass | 389.16 g/mol |
MOTW update
Clothianidin and thiamethoxam were the Molecules of the Week for February 17, 2014 and October 15, 2012, respectively. Both are neonicotinoid insecticides that during the past decade have come under regulatory pressure because they are toxic to honeybees. Finally, this past week, the US Environmental Protection Agency canceled the registrations for 12 formulations that contain one or the other of these pesticides.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

