What molecule am I?
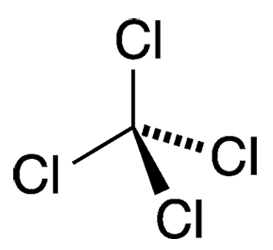
Readers of a certain age can remember when “carbon tet” was the go-to household solvent that easily removed organic residues such as adhesives and oils from almost any surface. It was also a widely used dry-cleaning solvent, refrigerant, and fire suppressant.
During the 1970s and ’80s, however, toxicologists discovered that inhalation of or skin contact with carbon tetrachloride (CCl4) can damage many organs, including the liver, kidneys, and central nervous system (see hazard table). CCl4 is also a “greenhouse gas” that, according to the 1995 Montreal Protocol, depletes ozone in the Earth’s stratosphere. Consequently, production and use have been significantly reduced.
In its heyday, CCl4 was produced by chlorinating C1 compounds such as chloroform, carbon disulfide, and, in later years, methane. What little is available today is a coproduct from the manufacture of chloroform and dichloromethane; however, US production is still 15 times the amount called for in the Montreal Protocol.
The CCl4 currently produced is used as a feedstock for ozone-safe refrigerants and in some minor agricultural and industrial processes. In a recent draft risk assessment of CCl4 standards under the Toxic Substances Control Act, the US Environmental Protection Agency reports that the compound is safe to use in manufacturing plants if workers wear appropriate personal protective equipment. The assessment is scheduled to be finalized by June.
Carbon tetrachloride hazard information
| Hazard class* | Hazard statement | |
|---|---|---|
| Acute toxicity, oral, category 3 | H301—Toxic if swallowed | |
| Acute toxicity, dermal, category 3 | H311—Toxic in contact with skin | |
| Skin sensitisation, category 1B | H317—May cause an allergic skin reaction | |
| Acute toxicity, inhalation, category 3 | H331—Toxic if inhaled | |
| Carcinogenicity, category 2 | H351—Suspected of causing cancer | |
| Specific target organ toxicity (liver, kidney), repeated exposure, inhalation, category 1 | H372—Causes damage to organs (liver, kidney) through prolonged or repeated exposure if inhaled | |
| Hazardous to the aquatic environment, acute hazard, category 3 | H402—Harmful to aquatic life | |
| Hazardous to the aquatic environment, long-term hazard, category 3 | H412—Harmful to aquatic life with long-lasting effects | |
| Hazardous to the ozone layer, category 1 | H420—Harms public health and the environment by destroying ozone in the upper atmosphere | |
*Globally Harmonized System of Classification and Labeling of Chemicals. Explanation of pictograms.
Carbon tetrachloride
fast facts
| CAS Reg. No. | 56-23-5 |
| SciFinder nomenclature | Methane, tetrachloro- |
| Empirical formula | CCl4 |
| Molar mass | 153.82 g/mol |
| Appearance | Colorless liquid |
| Boiling point | 77 ºC |
| Water solubility | 0.81 g/L |
MOTW Update
Vanillin was the Molecule of the Week for September 12, 2016. It is, of course, the major flavor component of vanilla. Most vanillin is made from petrochemical precursors, but now producers are seeking more sustainable sources. Siegfried R. Waldvogel and co-workers at Johannes Gutenberg University Mainz (Germany) report that by using an electrochemical reaction, they can produce vanillin and other useful compounds from lignin, a paper pulp byproduct.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

