What molecule am I?
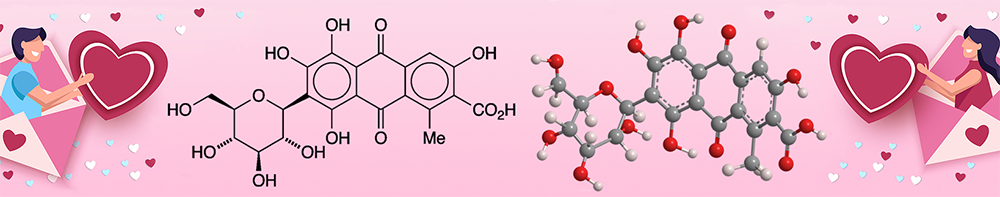
Carminic acid is a naturally occurring organic molecule whose structure consists of 9,10-anthraquinone-2-carboxylic acid “decorated” with a methyl group, a glucopyranose, and four hydroxyls. It is found in several species of scale insects known as cochineals, including Dactylopius coccus (Western Hemisphere), Porphyrophora hamelii (Armenia), and Porphyrophora polonica (north-central Europe).
In 1894, noted British dye chemist Henry Edward Schunk, working in Germany, isolated carminic acid from cochineals. German chemist Otto Dimroth reported its structure in 1920; and Indian chemists S. B. Bhatia and K. Venkataraman corrected the placement of the carboxyl group in Dimroth’s structure in 1965.
Cochineal insects use carminic acid to repel other insects. But for centuries, cochineals have been valuable to humans for providing carminic acid to use on its own or to make cochineal dyes. When carminic acid is treated with with aluminum and/or calcium salts, it forms complexes that are familiarly called carmine, carmine lake, crimson lake, or even the eponymous cochineal1. The dye is used for textiles and as a US Food and Drug Administration–approved food, cosmetic, and drug colorant, although some people exhibit allergic reactions to it.
Free carminic acid has been used as a bacteriological stain, an ingredient for artists’ paints, and a pigment for inks. In its cochineal dye form, you can even use it to make your own colored paper for cards and envelopes for Valentine’s Day!
1. CAS Reg. No. 1343-78-8.
Originally the Molecule of the Week for February 5, 2007
Carminic acid hazard information*
| Hazard class** | GHS code and hazard statement | |
|---|---|---|
| Acute toxicity, oral, category 4 | H302—Harmful if swallowed | |
| Serious eye damage/eye irritation, category 2A | H319—Causes serious eye irritation | |
Specific target organ toxicity, single exposure, respiratory tract irritation, category 3 | H335—May cause respiratory irritation | |
*Most safety data sheets state that carminic acid is not a hazardous substance or mixture. The information given here was found in only one SDS.
**Globally Harmonized System (GHS) of Classification and Labeling of Chemicals. Explanation of pictograms.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
Carminic acid
fast facts
| CAS Reg. No. | 1260-17-9 |
| SciFinder nomenclature | 2-Anthracene-carboxylic acid, 7-β-D-glucopyranosyl-9,10-dihydro-3,5,6,8-tetrahydroxy-1-methyl-9,10-dioxo- |
| Empirical formula | C22H20O13 |
| Molar mass | 492.39 g/mol |
| Appearance | Red crystals; bright red to dark purple powder |
| Melting point | 136 °C (dec.) |
| Water solubility | 1.3 g/L |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

