What molecule am I?
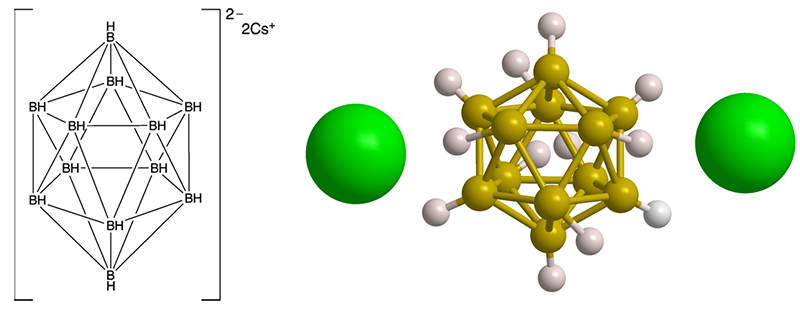
Cesium dodecaborate is one of many known salts of polyborane anions. The dodecaborate anion has the shape of a regular octahedron, one of the five platonic solids. Dodecaborate is isoelectronic with dodecahedrane, the Molecule of the Week for February 22, 2021.
In 1960, Anthony R. Pitochelli and M. Frederick Hawthorne1 at Rohm and Haas’s Redstone Arsenal Research Division (Huntsville, AL) prepared small amounts of some dodecaborate salts. The salts contained trimethylammonium, potassium, and triphenylmethylphosphonium cations.
Later in the decade, Henry C. Miller and Earl L. Muetterties at Du Pont (Wilmington, DE), developed a synthesis of the cesium salt. As described in the 1967 book Borane Anions2, the synthesis begins with the reaction of sodium borohydride (NaBH4) with boron trifluoride (BF3) to produce the triborate NaB3H8. The triborate is then pyrolyzed to give a mixture that includes sodium dodecaborate. The desired anion is obtained by precipitating it as the cesium salt.
Thomas Schlied and co-workers at the University of Stuttgart (Germany) published the crystal structure of cesium dodecaborate in 2000. The crystals are colorless, face-rich cubic with B–B distances of 178 pm and B–H distances of 112 pm. Each cesium ion is in contact with 12 hydrogen atoms, each at a distance of 313 pm.
In recent years, scientists have been exploring practical applications of dodecaborate salts, such as in battery electrodes, catalysis, and sensors. In particular, a research team led by Haibo Zhang and Xiaohai Zhou at the University of Wuhan (China) have used cesium dodecaborate to prepare complex catalysts.
In 2018, the researchers reported the preparation of a class of core–shell magnetic gold nanocomposites with “raspberry-like” structures. Cesium dodecaborate played a dual role in preparing highly monodispersed gold nanoparticles (AuNPs) and immobilizing them on the surface of a γCD@Fe3O4 substrate3. The resulting AuNPs@Fe3O4 composites made highly active and recyclable catalysts for the selective reduction of nitroaromatic compounds to the corresponding anilines.
Two years later, the group showed that cubic platinum nanoparticles capped with cesium dodecaborate catalyzes the direct oxidation of methane by hydrogen peroxide and oxygen to ethanol under mild (50 °C) conditions. The optimized reaction gave >97% conversion to ethanol.
1. Hawthorne, a giant in the field of boron chemistry, passed away last month at age 92. Nicknamed “Mr. Boron” by his associates, he was awarded the prestigious ACS Priestly Medal in 2009.
2. Hawthorne, Miller, and Muetterties, among others, were coauthors.
3. γCD@Fe3O4 is a γ-cyclodextrin–hematite complex.
Cesium dodecaborate hazard information
| Hazard class* | GHS code and hazard statement | |
|---|---|---|
| Flammable solids, category 1 | H228—Flammable solid | |
| Skin corrosion/irritation, category 2 | H315—Causes skin irritation | |
| Serious eye damage/eye irritation, category 2A | H319—Causes serious eye irritation | |
| Specific target organ toxicity, single exposure, respiratory tract irritation, category 3 | H335—May cause respiratory irritation | |
*Globally Harmonized System (GHS) of Classification and Labeling of Chemicals.
Explanation of pictograms.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
Cesium dodecaborate
fast facts
| CAS Reg. No. | 12008-75-2 |
| SciFinder nomenclature | Dodecaborate(2–), dodecahydro-, cesium (1:2) |
| Empirical formula | B12Cs2H12 |
| Molar mass | 407.64 g/mol |
| Appearance | White crystals or powder |
| Melting point | >650 °C |
| Water solubility | Slight |
Over the years, readers have noted that ionic substances are not actually molecules. This is correct, but we use "molecules" in the broadest sense to include them in Molecule of the Week.—Ed.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

