What molecule am I?

Chemists have long sought to identify stable all-nitrogen chemical species. N2, of course, is ubiquitous; and N3–, the azide anion, is well characterized. But what about N4 and higher?
Several studies, mostly theoretical, have been done in search of N4. In 1996, Mikhail N. Glukhovtsev at the University of Sydney and Sergei Laiter at the University of North Carolina (Chapel Hill) calculated that the energies of the three most likely N4 isomers increase in the order open-chain N4 triplet diradical < tetrazetetetrahedrane < planar tetrazete. The researchers attributed the higher energies of tetrahedral and planar N4 to ring strain and antiaromaticity, respectively. Note the reversal in the energies of the cyclic N4s versus those of the isoelectronic carbon systems tetrahedrane and cyclobutadiene.
In 2000, J. George Radziszewski and coauthors at the Colorado School of Mines and the National Renewable Energy Laboratory (both in Golden, CO), detected N4 in a nitrogen plasma and assigned its structure to tetrazetetetrahedrane.
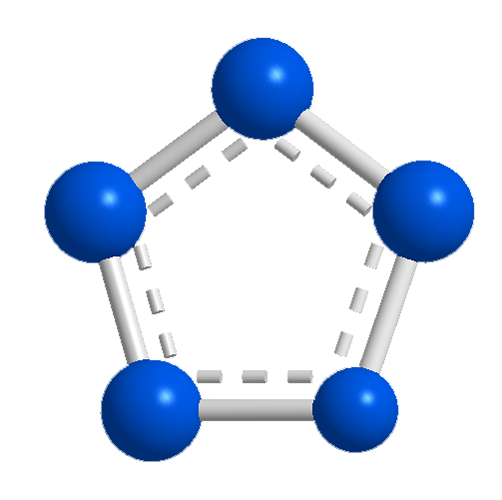
In another 1996 theoretical study, Glukhovtsev, Haijun Jiao, and Paul von Ragué Schleyer at the University of Erlangen-Nuremberg (Germany) and Rostov University (Rostov on Don, Russia) found that the most stable Nn compounds contain N5, or cyclopentazole, rings. An example is N8, azidopentazole. They attributed the stability to the aromaticity of the N5 rings.
This past year, the quest for a stable N5 species came to fruition. Yehuda Haas and colleagues at the Hebrew University of Jerusalem prepared the cyclopentazole anion, N5–, by reducing phenylpentazole with sodium metal. The sodium salt of the anion in tetrahydrofuran solution is indefinitely stable below –40 ºC and has a half-life of minutes at ambient temperature.
MOTW Update:
February 6, 2017
The cyclopentazole anion's sodium salt was the first stable N5compound ever synthesized. But this salt is stable only in aqueous solution. More recently, Chong Zhang, Chengguo Sun, and coauthors in China prepared a stable (but complex) crystalline cyclopentazolate salt: (N5)6(H3O)3(NH4)4Cl.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

