What molecules are we?
November is flavor and aroma month!
It’s almost Thanksgiving! Among the holiday treats that most of us enjoy is cranberry sauce, whether it’s full of whole berries or simply a wiggly gelatin. What compounds are in cranberries that make the sauce taste so good?
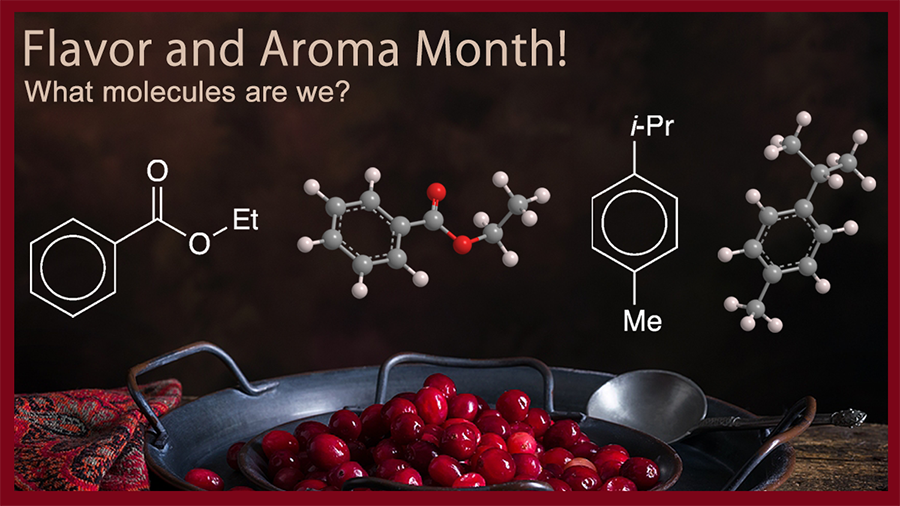
According to the Science and Food program at the University of California, Los Angeles, the primary flavor compounds are ethyl benzoate, p-cymene, α-terpineol, and 1,8-cineole (aka eucalyptol). The latter two are former Molecules of the Week, so the first two take center stage this year.
Ethyl benzoate is a simple ester of benzoic acid with a sweet, fruity aroma. It has been known since the late 19th century; it is found in fruits such as apples, bananas, cherries, and of course, cranberries. Synthetic ethyl benzoate is made by esterifying benzoic acid and ethanol with an acid catalyst such as hydrochloric or sulfuric acid. It is used as an ingredient in perfumes and artificial fruit-flavoring agents.
p-Cymene is a dialkyl aromatic hydrocarbon. In addition to being a flavor component in cranberries, it is abundant in several essential oils, notably the oils of cumin and thyme. Its aroma and flavor have been described as having hints of citrus, earth, and wood. It can be synthesized from petrochemicals (e.g., the alkylation of toluene with propylene) or from natural terpenes (e.g., by microwave irradiation of limonene). Like ethyl benzoate, p-cymene is used as a flavoring agent; but it also has medicinal uses (in cough syrup) and as a starting material for manufacturing pesticides.
Needless to say, while you’re enjoying your Thanksgiving dinner, you won’t be focusing on any of this. Have a happy holiday!
Ethyl benzoate hazard information
| Hazard class* | GHS code and hazard statement | |
|---|---|---|
| Flammable liquids, category 4 | H227—Combustible liquid | |
| Short-term (acute) aquatic hazard, category 2 | H401—Toxic to aquatic life | |
p-Cymene hazard information**
| Hazard class* | GHS code and hazard statement | |
|---|---|---|
| Flammable liquids, category 3 | H226—Flammable liquid and vapor | |
| Aspiration hazard, category 1 | H304—May be fatal if swallowed and enters airways | |
| Skin corrosion/irritation, category 2 | H315—Causes skin irritation | |
| Serious eye damage/eye irritation, category 2A | H319—Causes serious eye irritation | |
| Specific target organ toxicity, single exposure, respiratory tract irritation category 3 | H335—May cause respiratory irritation | |
| Reproductive toxicity, category 2 | H361—Suspected of damaging fertility or the unborn child | |
| Short-term (acute) aquatic hazard, category 2 | H401—Toxic to aquatic life | |
| Long-term (chronic) aquatic hazard, category 2 | H411—Toxic to aquatic life with long-lasting effects | |
*Globally Harmonized System (GHS) of Classification and Labeling of Chemicals. Explanation of pictograms.
**Compilation of selected safety data sheets.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
Ethyl benzoate fast facts
| CAS Reg. No. | 93-89-0 |
| SciFinder nomenclature | Benzoic acid, ethyl ester |
| Empirical formula | C9H10O2 |
| Molar mass | 150.17 g/mol |
| Appearance | Colorless liquid |
| Boiling point | 212° C |
| Water solubility | 0.7 g/L |
p-Cymene fast facts
| CAS Reg. No. | 99-87-6 |
| SciFinder nomenclature | Benzene, 1-methyl-4-(1-methylethyl)- |
| Empirical formula | C10H14 |
| Molar mass | 134.22 g/mol |
| Appearance | Colorless liquid |
| Boiling point | 177° C |
| Water solubility | 23 mg/L |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

