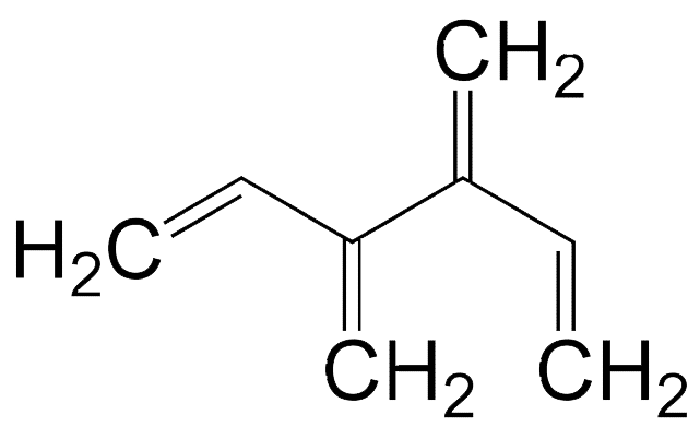
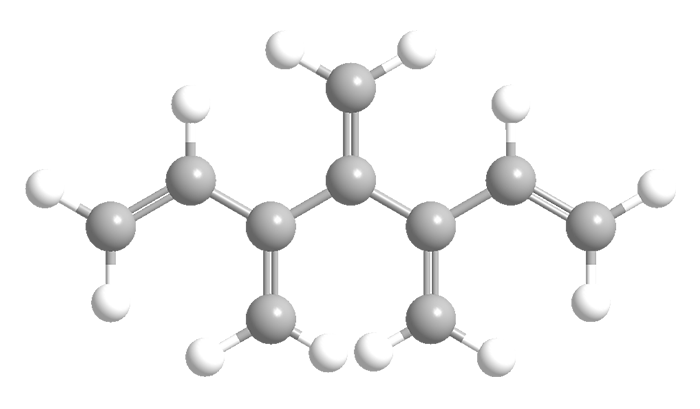
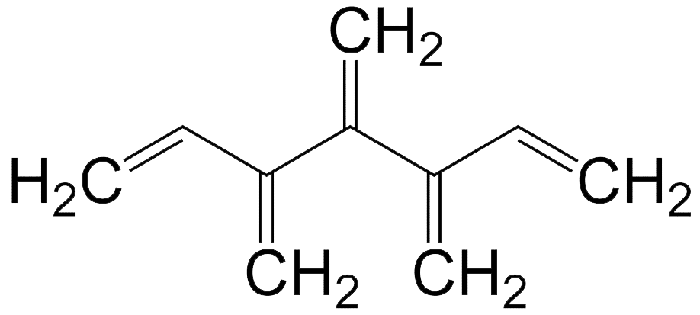

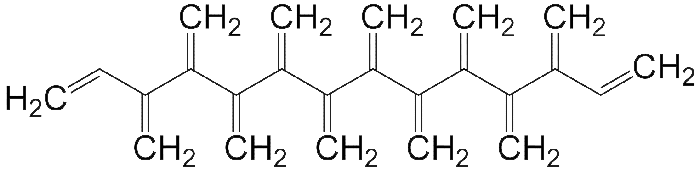
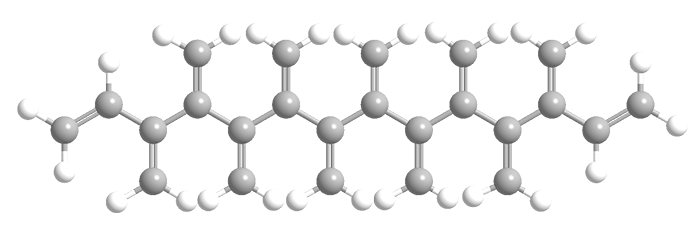
Like radialenes, last week’s molecules, dendralenes are polyolefins that are studied mainly for their synthetic methods and bonding properties. They consist of straight-chain alkanes that have an exo methylene group attached to each carbon atom. Thus, in all except the simplest one (butadiene), the double bonds are cross-conjugated.
Dendralenes are named for the number of double bonds they contain. The ones shown here are [4]-, [5]-, and [12]dendralene.
Up until this year, only the syntheses of dendralenes [3] through [8] had been reported. The earliest, [3]dendralene, was made in 1955. Now, chemists Michael S. Sherburn, Michael N. Paddon-Row, and colleagues at Australian National University (Canberra) and the University of New South Wales (Sydney)—the same researchers who synthesized [5]radialene—report the syntheses of dendralenes [9] through [12].
Like other olefins with conjugated double bonds, dendralenes readily undergo Diels–Alder cyclizations to produce complex molecules with additional double bonds that are available for creating even larger structures. Dendralenes can also undergo multiple insertions of methylene groups with reagents such as dibromomethane or diazomethane–zinc iodide to produce oligocyclopropanes.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

