What molecule am I?
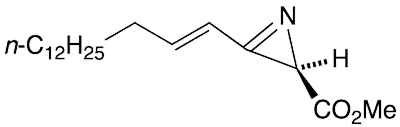
(R)-Dysidazirine is a natural product that Tadeusz F. Molinski and Chris M. Ireland* at the University of Utah (Salt Lake City) discovered in 1988 in the marine sponge Dysidea fragilis. The 2H-azirine ring occurs in nature only in D. fragilis, congeners from a different sponge, and azirinomycin1, an antibiotic that researchers at Merck (Rahway, NJ) obtained in 1970 by culturing the bacterium Streptomyces aureus.
Remarkably, despite its exceptionally high ring strain, the α,β-unsaturated 2H-azirine ring is stable in the absence of nucleophiles. The 1H NMR spectrum of a sample stored at –20 °C for 12 years remained unchanged, but the molecule had partially racemized.
Franklin Davis*, G. Venkat Reddy, and Hu Liu at Temple University (Philadelphia) reported the synthesis of optically pure (R)-dysidazirine in 1995. The data for the synthetic compound and natural product perfectly matched. except for the specific rotation. Apparently, the natural (R)-dysidazirine was only 89% optically pure.
Dysidazirine exhibits potent in vitro antifungal activity against the pathogenic yeast Candida albicans. It also inhibits cultured HCT-116 human colon cancer cells.
No hazard information on (R)-dysidazirine is available.
MOTW updates
Aspirin1 was the Molecule of the Week for June 4, 2012. It is a venerable pain remedy and anticlotting agent that for decades has been recommended by physicians in low doses (81 mg) as a prophylactic against the risk of heart attack and stroke.
But recently, the independent US Preventive Services Task Force (USPSTF) reversed this recommendation for patients older than 60 years who have no history of heart attack or stroke. The USPTF stated that these patients receive no benefit from aspirin but may be at risk of internal bleeding from the compound. Some physicians, like Abbas Bitar at the University of Michigan, say that higher-risk patients should still consider a regimen of low-dose aspirin.
Bismuth subsalicylate2 was the Molecule of the Week for November 28, 2005. It is the active ingredient in more than century-old antiacid formulations, such as Pepto-Bismol, for “upset stomach”—nausea, indigestion, and the like.
Despite its long use, the crystal structure of bismuth subsalicylate was unknown until recently. Tom Willhammar, A. Ken Inge, and fellow researchers at Stockholm University used 3-D electron diffraction and scanning transmission electron microscopy to discover that the molecule has a layered structure with multiple stacking orientations. The stacking variations are the reason that the structure determination was so challenging.
1. CAS Reg. No. 50-78-2.
2. CAS Reg. No. 14882-18-9.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
(R)-Dysidazirine fast facts
| CAS Reg. No. | 113507-74-7 |
| SciFinder nomenclature | 2H-Azirine-2-carboxylic acid, 3-(1E)-1- pentadecen-1-yl-, methyl ester, (2R)- |
| Empirical formula | C19H33NO2 |
| Molar mass | 307.47 g/mol |
| Appearance | Low-melting white solid |
| Melting point | Not reported |
| Water solubility | Sparingly soluble; specific solubility not reported |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

