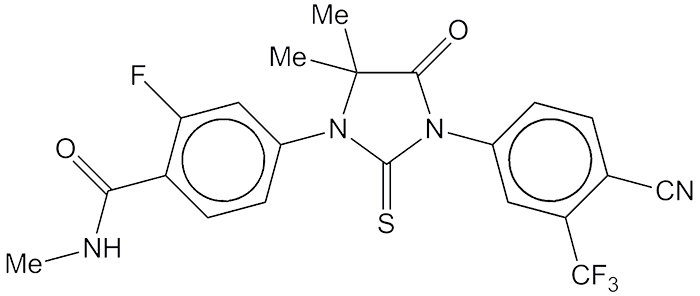
At the 2008 ACS Fall National Meeting, chemist M. E. Jung and his research group at UCLA, along with oncologist C. L. Sawyers, reported a new drug called RD162 that showed activity against refractory metastatic prostate cancer. RD162 is one of a then-new class of prostate cancer drugs that targets the androgen receptor.
Under a 2005 agreement with UCLA, San Francisco drug company Medivation licensed and developed an analogue of RD162 that they called MCV3100. In 2012, the US Food and Drug Administration approved MCV3100 for treating prostate cancer under the generic name enzalutamide. Medivation markets the drug under the trade name Xtandi.
Nice story, right? But it has a nasty side. In 2009, Jung and Sawyers formed Aragon Pharmaceuticals to develop similar molecules not licensed to Medivation. The drug company sued UCLA on the grounds that it wasn’t offered the nonlicensed compounds; but in 2012, a judge decided in UCLA’s favor.
But wait, there’s more. In April 2014, UCLA sued Medivation, claiming that the 2005 agreement entitles the university to 10% of the “operating profits” from Xtandi. Medivation denied the claim and says it intends to contest it vigorously. And the beat goes on.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

