What molecule am I?
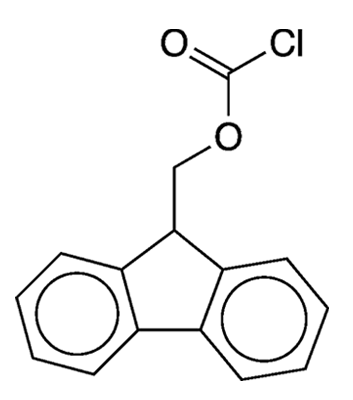
Protecting groups are a fundamental tool in organic synthesis. In general, a protecting group is used to “protect” an otherwise labile or reactive functional group from interfering in subsequent steps of a synthesis. When this protection is no longer needed, a suitable reagent is used to “deprotect” the protected moiety.
Amines constitute one of the more common functional groups that need to be protected during a synthesis. In 1970, Louis A. Carpino* and Grace Y. Han at the University of Massachusetts (Amherst) noted, “In contrast to the variety of amino-protecting groups [that] can be cleaved . . . by acids of varying strengths, there is currently no complementary set of groups cleavable by basic reagents of graded activity.” They went on to introduce the 9-fluorenylmethyloxycarbonyl (Fmoc) group that, once no longer needed, can be removed under basic conditions.
The reagent the researchers chose to install the Fmoc group was 9-fluorenylmethyloxycarbonyl chloride (Fmoc-Cl), also frequently called 9-fluorenylmethyl chloroformate. They prepared Fmoc-Cl by treating 9-fluorenylmethanol with phosgene (COCl2). As an alternative to Fmoc-Cl, they prepared the corresponding 9-fluorenylmethyl azidoformate, which they made via the reaction of Fmoc-Cl with sodium azide (NaN3).
In an initial example of amine protection/deprotection, Carpino and Han treated glycine with Fmoc-Cl. They then showed that treating the resulting Fmoc derivative with liquid ammonia quantitatively releases the original amine, along with the easily separable byproducts carbon dioxide and dibenzofulvalene.
In a subsequent publication, Carpino and Han discussed the shortcomings of other protecting groups that they examined before they came upon the Fmoc group. They also provided numerous examples of Fmoc-protected amino acids, amino esters, and dipeptides. Finally, they demonstrated that strong organic amines such as piperidine, morpholine, and ethanolamine can be used in place of ammonia to remove Fmoc.
Carpino and Han went on to patent Fmoc-Cl and other Fmoc derivatives as compounds useful in peptide synthesis and for separating optical isomers (US Patent 3,835,175, Sept. 10, 1974). A subsequent patent (US Patent 3,906,031, Sept. 16, 1975) claimed Fmoc-protected amino acids, particularly phenylalanine. Both patents were assigned to Research Corp. (New York).
9-Fluorenylmethyloxycarbonyl chloride
hazard information
| Hazard class** | Hazard statement | |
|---|---|---|
| Corrosive to metals, category 1 | H290—May be corrosive to metals | |
| Acute toxicity, oral, category 4 | H302—Harmful if swallowed | |
| Acute toxicity, dermal, category 4 | H312—Harmful in contact with skin | |
| Skin corrosion/irritation, category 1B | H314—Causes severe skin burns and eye damage | |
| Serious eye damage/eye irritation, category 1 | H318—Causes serious eye damage | |
| Acute toxicity, inhalation (dusts/mists), category 4 | H332—Harmful if inhaled | |
| Specific target organ toxicity, single exposure, respiratory tract irritation, category 3 | H335—May cause respiratory irritation | |
*Compilation of multiple safety data sheets.
**Globally Harmonized System of Classification and Labeling of Chemicals.
Explanation of pictograms.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
9-Fluorenylmethyloxycarbonyl chloride fast facts
| CAS Reg. No. | 28920-43-6 |
| SciFinder nomenclature | Carbonochloridic acid, 9H-fluoren-9-ylmethyl ester |
| Empirical formula | C15H11ClO2 |
| Molar mass | 258.70 g/mol |
| Appearance | White to off-white crystalline powder |
| Melting point | 62–64 ºC |
| Water solubility | Insoluble; decomposes |
MOTW update
Prussian blue was the Molecule of the Week for January 23, 2017. It is a pigment that is used to color paints, inks, textiles, and other commercial products. But during the past decade, Prussian blue found a high-tech use: Its ability to transfer electrons efficiently makes it an ideal substance for use in sodium-ion battery electrodes. Battery producer Natron Energy (Santa Clara, CA) recently concluded a deal with Lonza Group (Basel, Switzerland) to supply 700–1000 t/year of Prussian blue for the Natron BlueTray 4000 battery system to be used for data-center and telecommunications applications.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

