What molecule am I?
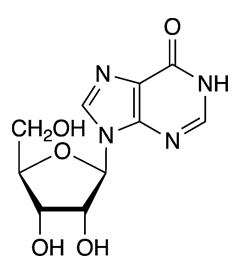
Inosine is a natural purine nucleoside that commonly occurs in transfer RNAs in humans. The molecule consists of hypoxanthine connected to a ribofuranose ring via a glycosidic bond. It is a degradation product of adenosine.
Elsewhere in nature, inosine is found in red meat, pork, and poultry. Its 5’-monophosphate is associated with the flavor and taste of red and white meats.
In 1910 chemists Phoebus A.T. Levene and Walter A. Jacobs at the Rockefeller Institute of Medical Research in New York City (now Rockefeller University) synthesized inosine by treating adenosine with sodium nitrate and acetic acid. Later (in 1947), using less harsh conditions, Herman M. Kalckar at the Public Health Institute of the City of New York prepared inosine by incubating adenosine with intestine-based adenosine deaminase.
Since 2000, inosine has been tried as a treatment for medical conditions such as Parkinson’s disease, multiple sclerosis, stroke, and spinal cord injuries. None of these efforts produced a commercial drug. But earlier this year, inosine turned up in an off-target result generated by a CRISPR-based gene editor.
David R. Liu and collaborators at the Broad Institute of Harvard and MIT (Cambridge, MA) and Harvard University found that an adenine base editor (ABE) that efficiently converts targeted A–T base pairs in genomic DNA to G–C pairs does not do so well when it edits cellular RNAs. The editor, called ABEmax, generates a low but significant amount of inosine from the adenosine in RNA. The authors do not know whether the error will have any biological effects. But they note that some cancers evaded immune checkpoint inhibitors when similar results occurred in other RNA studies.
Inosine hazard information
| GHS classification*: not a hazardous substance or mixture |
*Globally Harmonized System of Classification and Labeling of Chemicals.
MOTW update: June 26, 2023
Adenosine and inosine were the Molecules of the Week for June 5, 2010, and July 15, 2019, respectively. They are nucleosides that differ only by the hydroxyl configurations on the ribofuranose ring. In 2019, researchers found that a gene editor unexpectedly converted a small amount of adenosine in an RNA to inosine.
Earlier this month, Eli Eisenberg and collaborators at Tel Aviv University, the University of Connecticut Health Center (Farmington), Bar-Ilan University (Ramat Gan, Israel), and the University of Puerto Rico (San Juan) reported that this conversion can be advantageous to squids and other cephalopods. The researchers found that seawater temperature decreases induce the cephalopods to self-edit mRNA adenosine to inosine. The nucleoside transformation enables the animals to prevent low-temperature “brain freeze”.
Inosine fast facts
| CAS Reg. No. | 58-63-9 |
| Empirical formula | C10H12N4O5 |
| Molar mass | 268.23 g/mol |
| Appearance | White crystals or powder |
| Melting point | 218–226 ºC (dec.)a |
| Water solubility | 16 g/L |
a. Decomposition temperature depends on heating rate.
MOTW update
Fentanyl was the Molecule of the week for December 11, 2017. It is a widely abused opioid painkiller. The opioid antidote naloxone is not completely effective against overdoses of fentanyl and its companion drug carfentanyl; but recently Kim D. Janda and colleagues at the Scripps Research Institute (La Jolla, CA) developed monoclonal antibodies that blunt the effects of these drugs by sequestering them in the bloodstream.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

