
The elements in group 18 of the periodic table (the “noble gases”) were once considered to be chemically inert. But over the years, chemists discovered how to make molecules that contain them.
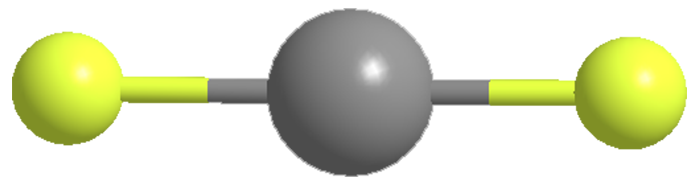
In the case of krypton, J. J. Turner and G. C. Pimentel at the University of California, Berkeley, used electrical discharge to prepare krypton difluoride (KrF2) in 1963. The colorless solid decomposes at room temperature, but it can be stored indefinitely at –78 ºC.
KrF2 is an extremely strong oxidizing and fluorinating agent. It can convert metallic gold to AuF5, metallic silver to AgF3, and xenon to XeF6. It can also oxidize chlorine and bromine to their +5 oxidation states.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

