What molecule am I?

Among the “holy grails” in chemistry and physics is the development of practical superconductive materials. A substance is a superconductor if it exhibits no electrical resistance when it is cooled to below a certain temperature, called the critical transition temperature or Tc.
Superconductivity was discovered by Dutch physicist Heike Kamerlingh Onnes in 1911. He was awarded the Nobel Prize in Physics for this and other discoveries in 1913. To attain superconductivity, Kamerlingh Onnes found that he had to cool pure metals to within a few degrees of absolute zero (0 K or –273.16 ºC).
Since 1911, researchers have made materials with increasingly higher Tc values. By 2015, a hydrogen–sulfur compound that was formed under high pressure set the high-Tc record of –70º C, still too low for practical applications.
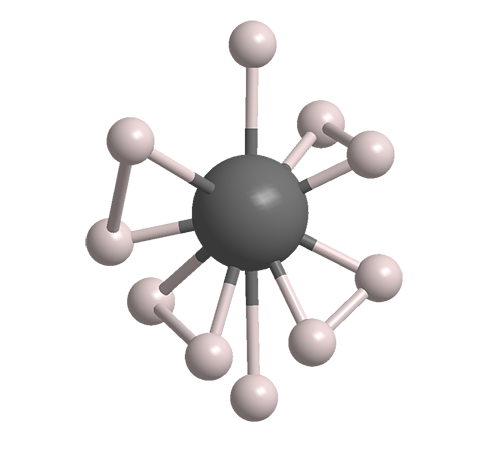
But just last month, the record was broken again. Maddury Somayazulu, Russell J. Hemley, and colleagues at George Washington University (Washington, DC), Carnegie Institution of Washington (DC), and Argonne National Laboratory (IL) made a compound with the empirical formula LaH101 that exhibited a “hot” Tc of –13 ºC. This temperature is almost high enough to be practical; but the catch is that LaH10, or lanthanum decahydride, is stable only at the pressure at which it is formed: ≈200 GPa or about 2 million times the Earth’s atmospheric pressure at sea level.
Now that superconductivity near ambient temperature has been achieved, the next step presumably will be to find a way to achieve similarly high-Tc materials that are stable at usable pressures.
1. The images shown here are for illustrative purposes only; the exact structure of LaH10 has yet to be established.
Lanthanum decahydride fast facts
| CAS Reg. No. | 1130489-79-0 |
| Empirical formula | H10La |
| Molar mass | 148.98 g/mol |
| Appearance | Crystalline solid |
| Melting point | Unstable |
| Water solubility | Unstable |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

