What molecule am I?
November is flavor and aroma month at MOTW!
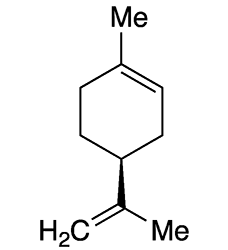
Limonene is a monoterpene that exists in nature in two enantiomers: (S)-limonene [aka (–)-limonene, L-limonene] and (R)-limonene [aka (+)-limonene, D-limonene]. The (S)-isomer is shown here.
Both enantiomers have well-recognized flavors and aromas. (R)-Limonene1 is found in citrus oils and has the flavor and fragrance of oranges. (S)-Limonene is produced by coniferous trees and caraway, dill, and bergamot plants; its piny odor contributes to the flavors and aromas of the plants’ edible portions.
In addition to their commercial use as food flavorings, the limonenes are used in industrial cleaning solvents, wetting agents, air fresheners, and fragrances in personal care products. Worldwide production is ≈50,000 t/year with a 2020 market value of US$323 million.
Compounds like the limonenes were recently reported to have a downside. In a study conducted in 2018, Matthew Coggon and co-workers at the National Oceanic and Atmospheric Administration (Boulder, CO) collected air samples from various locations in New York City and analyzed the samples for volatile organic compounds (VOCs), which are notorious ozone generators.
The results, published in August 2021, showed that half of the VOCs were produced by fossil fuel combustion, an expected culprit for creating ozone. The other half came from chemical products, including solvents for coatings and adhesives, also typical ozone precursors. But fully half of the identified chemicals consisted largely of monoterpenes, such as limonene, and other substances used in personal care products. The study provides new clues for potential control of ozone formation in densely populated areas.
On the more positive side, (S)-limonene was in the news last month as an intermediate in an engineered biosynthetic process to make an unnatural terpene. John Hartwig and colleagues at the University of California, Berkeley, and Lawrence Berkeley National Laboratory created a strain of Escherichia coli to produce (S)-limonene, a Sulfolobus acidocaldarius2 enzyme, and a porphyrin-specific transporter protein inside its cells.
In a medium that contains an iridium mesoporphyrin and ethyl diazoacetate, the engineered E. coli imbibes the porphyrin, where it combines with the enzyme to promote an insertion reaction between the limonene and the diazo compound to form a cyclopropylterpene ester. The process mimics natural terpene-modification reactions with high stereoselectivity.
The limonenes should not be confused with limonin3, a bitter, highly complex citrus product, or lemonene, an obscure name for the aromatic compound biphenyl,4 which occurs naturally in coal tar and crude oil.
1. CAS Reg. No. 5989-27-5.
2. Sulfolobus acidocaldarius is a single-cell organism that grows at high temperature and acidity.
2. CAS Reg. No. 1180-71-8.
3. CAS Reg. No. 92-52-4.
(S)-Limonene hazard information*
| Hazard class** | GHS code and hazard statement | |
|---|---|---|
| Flammable liquids, category 3 | H226—Flammable liquid and vapor | |
| Aspiration hazard, category 1 | H304—May be fatal if swallowed and enters airways | |
| Skin corrosion/irritation, category 2A | H315—Causes skin irritation | |
| Skin sensitization, category 1 | H317—May cause an allergic skin reaction | |
| Serious eye damage/eye irritation, category 2 | H319—Causes serious eye irritation | |
| Short-term (acute) aquatic hazard, category 1 | H400—Very toxic to aquatic life | |
*Compilation of selected safety data sheets.
**Globally Harmonized System (GHS) of Classification and Labeling of Chemicals. Explanation of pictograms.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
(S)-Limonene fast facts
| CAS Reg. No. | 5989-54-8 |
| SciFinder nomenclature | Cyclohexene, 1-methyl-4-(1-methylethenyl)-, (4S)- |
| Empirical formula | C10H16 |
| Molar mass | 136.23 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Boiling point | 177–178° C |
| Water solubility | 14 mg/L |
MOTW update
EIDD-2801 was the Molecule of the Week for June 1, 2020. We reported that researchers at Emory University (Atlanta) and other institutions modified an existing antiviral compound and found that it was effective, first against the original SARS-CoV, and then against SARS-CoV-2, the virus responsible for COVID-19. The US Food and Drug Administration approved an investigational new drug application for EIDD-2801 in near-record time.
Flash forward 18 months: The molecule, now known as molnupiravir, is being developed by Merck (Kenilworth, NJ). In October, Merck reported that molnupiravir cuts the risk of hospitalization and death from COVID-19 by 50% in people who have mild to moderate forms of the disease. The company submitted an emergency use authorization application for molnupiravir to FDA on October 11.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

