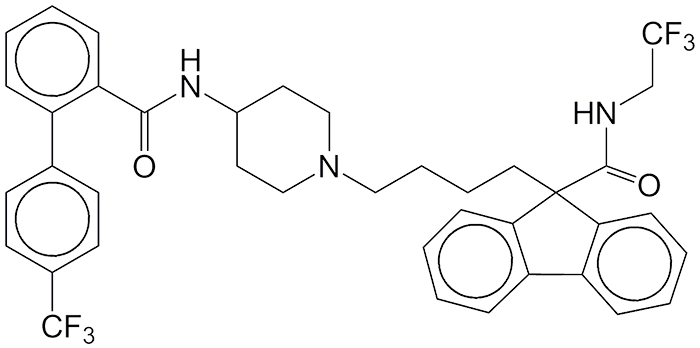
The drug lomitapide was originally developed to reduce low-density lipids (“bad” cholesterol) but failed in Phase I clinical trials. M. Beer and colleagues at Aegerion Pharmaceuticals (Cambridge, MA), however, “rescued” lomitapide by developing it as a treatment for the orphan disease homozygous familial hypercholesterolemia (HoFH), a genetic disorder that affects the liver and often causes heart failure. Lomitapide inhibits the microsomal triglyceride transfer protein needed for the assembly of bad cholesterol and its secretion in the liver. Aegerion and its pharmaceutical services partner, Aptuit (Greenwich, CT), rushed lomitapide through trials; and the US FDA approved as an HoFH drug.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

