What molecule am I?
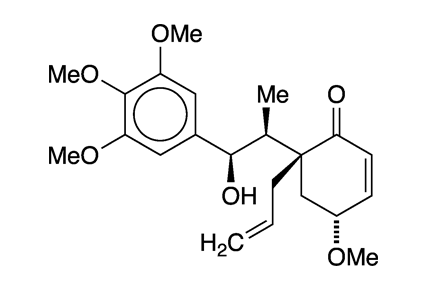
Every so often at Molecule of the Week, we come across a molecule with a funny or unusual name. So, this week we give you megaphone.
How did a molecule come to be named after a bullhorn? Actually, it didn’t. Megaphone takes its name from Aniba megaphylla, a South American tree in the Lauraceae (laurel) family. The compound, with four methoxy groups, one hydroxyl, and one ketone moiety, is considered to have a neolignan structure, that is, a molecule that consists of two n-propylbenzene units joined at the benzene rings.
In 1978, in a series of articles on tumor inhibitors, Kenneth L. Stevens, Albert T. Sneden, Robert F. Bryan, and co-workers at the University of Virginia (Charlottesville) reported that an alcohol extract from A. megaphylla root showed in vitro inhibitory activity against human carcinoma cells. They isolated and identified megaphone and two more cytotoxins from the extract.
Subsequent articles until 1993 focused on the total synthesis of megaphone. But alas, megaphone and its cohorts faded away in the literature. Very few properties and no hazard information have been reported. All that’s left is its funny name.
Megaphone fast facts
| CAS Reg. No. | 64332-37-2 |
| Empirical formula | C22H30O6 |
| Molar mass | 390.48 g/mol |
| Appearance | Crystalline solid |
| Melting point | 152 ºC |
| Water solubility | Not reported; likely insoluble |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

