What molecule am I?
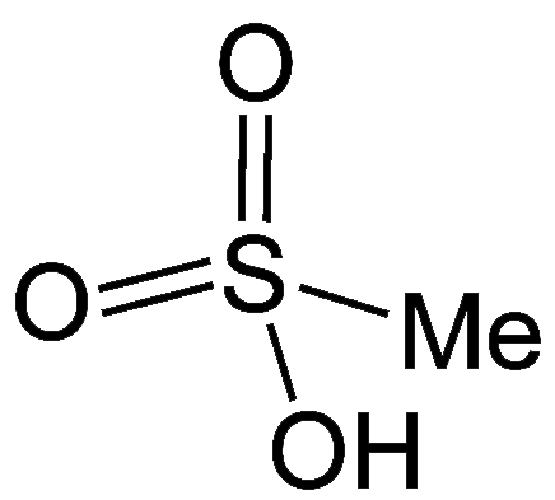
Methanesulfonic acid (MSA), the simplest alkanesulfonic acid, is a hygroscopic colorless liquid or white solid, depending on whether the ambient temperature is greater or less than 20 ºC. It is very soluble in water and oxygenated solvents, but sparingly soluble in most hydrocarbons. In aqueous solution, it is a strong acid (completely ionized).
MSA’s acidity and solubility properties make it industrially valuable as a catalyst in organic reactions, particularly polymerization. In many applications, its advantage over concentrated sulfuric acid is that it has similar acid strength but is not an oxidant.
The first report of MSA synthesis was in a 1950 patent awarded to John C. Snyder and Aristid V. Grosse of Houdry Process Corp. (subsequently acquired by Air Products). They heated methane and sulfur trioxide to 200–325 ºC under pressure in the presence of a mercury catalyst. BASF currently produces the acid via a two-step process in which methanol and elemental sulfur react to give dimethyl disulfide, which is then oxidized to the final product.
For decades, chemists tried to find a way to prepare methanesulfonic acid from methane and sulfur trioxide under much milder conditions than were used in the original method. In 2015, Grillo-Werke (Duisburg, Germany), in a world patent application, described the preparation of alkanesulfonic acids from alkanes and SO3 in the presence of an organic peroxide at temperatures up to 65 ºC and pressures up to 11 MPa. In June of this year, Grillo announced plans to compete with BASF by building a plant to produce MSA with a process that is likely based on that patent.
Grillo believes that its process will increase the supply of MSA so that it can be used for more applications. The company says that the plant should be operating by 2019

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

