What molecule am I?

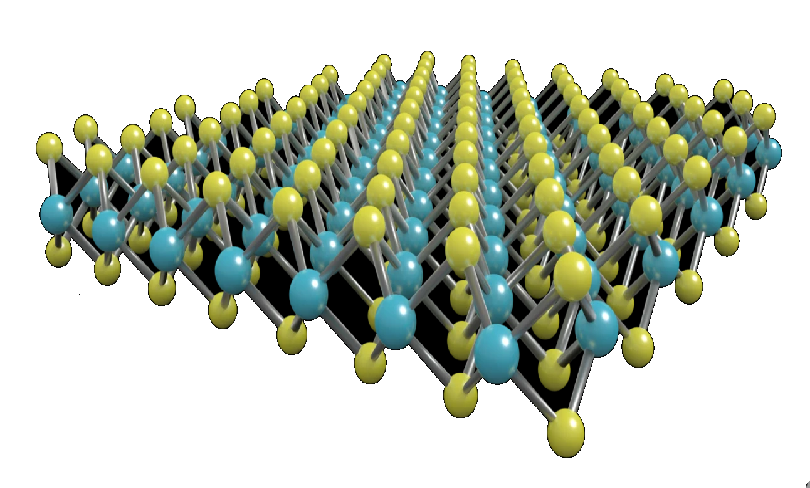
Molybdenum disulfide [molybdenum(IV) sulfide, MoS2] is an inorganic compound that exists in nature in the mineral molybdenite. Its crystals have a hexagonal layered structure (shown) that is similar to graphite.
In 1957, Ronald E. Bell and Robert E. Herfert at the now-defunct Climax Molybdenum Company of Michigan (Ann Arbor) prepared what was then a new rhombohedral crystalline form of MoS2. Rhombohedral crystals were subsequently discovered in nature.
Like most mineral salts, MoS2 has a high melting point, but it begins to sublime at a relatively low 450 ºC. This property is useful for purifying the compound.
Because of its layered structure, hexagonal MoS2, like graphite, is an excellent “dry” lubricant. It and its cousin tungsten disulfide can be used as surface coatings on machine parts (e.g., in the aerospace industry), in two-stroke engines (the type used for motorcycles), and in gun barrels (to reduce friction between the bullet and the barrel).
Unlike graphite, MoS2 does not depend on adsorbed water or other vapors for its lubricant properties. It can be used at temperatures as high as 350 ºC in oxidizing environments and up to 1100 ºC in nonoxidizing environments. Its stability makes it useful in high-temperature applications in which oils and greases are not practical.
In addition to its lubricating properties, MoS2 is a semiconductor. It is also known that it and other semiconducting transition-metal chalcogenides become superconductors at their surfaces when doped with an electrostatic field.
The mechanism of superconductivity was uncertain until 2018, when Andrea C. Ferrari at the University of Cambridge (UK) and colleagues there and at the Polytechnic Institute of Turin (Italy) reported that a multivalley Fermi surface is associated with the superconductivity state in MoS2. The authors believe that “this [Fermi surface] topology will serve as a guideline in the quest for new superconductors.”
Molybdenum disulfide hazard information
| Hazard class* | Hazard statement | |
|---|---|---|
| Acute toxicity, inhalation, category 4 | H332—Harmful if inhaled | |
*Globally Harmonized System of Classification and Labeling of Chemicals.
Explanation of pictograms.
Molybdenum disulfide
fast facts
| CAS Reg. No. | 1317-33-5 |
| SciFinder nomenclature | Molybdenum sulfide (MoS2) |
| Empirical formula | MoS2 |
| Molar mass | 160.07 g/mol |
| Appearance | Black or lead-gray crystals or powder |
| Boiling point | 2375 ºC |
| Water solubility | Insoluble |
MOTW update:
October 11, 2021
Molybdenum disulfide (MoS2) is an excellent “dry” lubricant, a semiconductor, and an important molecule in superconductivity research. Now, Jiwoong Park at the University of Chicago, David Cahill at the University of Illinois at Urbana–Champaign, and Paul Erhart at the Chalmers University of Technology (Gothenburg, Sweden) report that stacked sheets of MoS2 form a strong thermal conductor in two dimensions but block heat transfer between layers. The 900-fold heat-conduction ratio breaks the record set by single-crystal graphite by a factor of 3.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

