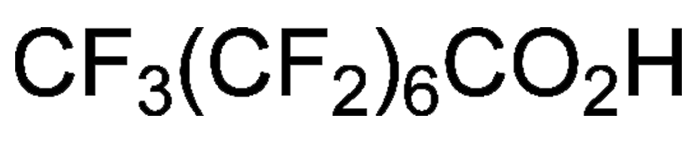
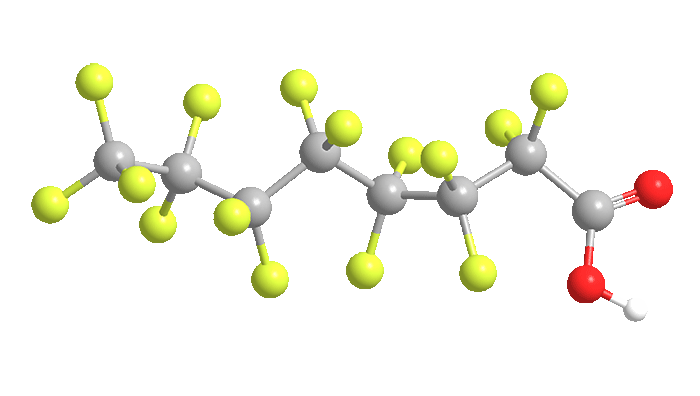
Perfluorooctanoic acid (PFOA), or pentadecafluorooctanoic acid, is a colorless liquid with a boiling range of 189–192 ºC. Its chief use is as a surfactant in the emulsion polymerization of fluoropolymers such as poly(tetrafluoroethylene) (Teflon). It is invaluable in the production of microchips.
In 1947, the 3M Company began to manufacture PFOA via electrochemical fluorination. Octanoyl chloride is treated with hydrofluoric acid, which replaces all of the hydrogen atoms with fluorine; and the acyl fluoride product is then hydrolyzed to PFOA. PFOA also can be produced in a telomerization process, in which smaller fluorocarbons react to make larger ones.
The C–F bonds in PFOA make it quite chemically inert, which turns out to be a blessing and a curse. Its inertness makes it persistent in the environment; it has been detected, mostly at very low levels, in the blood of ≈98% of the US population. Because it is carcinogenic and exhibits other forms of toxicity, governments have restricted the concentrations permitted in drinking water and are threatening to ban it altogether. As PFOA and similar fluorinated compounds become more restricted, electronics companies are struggling to replace them in the manufacture of ever-smaller semiconductor chips.
MOTW update
PFOA Suit Goes to Trial
DuPont, a long-time manufacturer of PFOA at its Parkersburg, WV, plant, and its successor, Chemours, are being sued by residents who claim that the chemical made them ill. The plaintiffs allege that PFOA that leaked into the area’s drinking water caused various diseases, including some cancers.
About 3500 lawsuits have been filed, of which six have been selected as bellwether suits. The first of these went to trial in September 2015. The results of the six suits will determine the course that plaintiffs and defendants will take in future cases.
MOTW update:
February 12, 2018
Perfluorooctanoic acid (PFOA) is widely used in the production of polytetrafluoroethylene (Teflon) and microchips, but it is being phased out because it is a significant contaminant in drinking water.
In 2010, DuPont introduced GenX, another perfluorinated surfactant, as a “sustainable” replacement for PFOA. But water in the river downstream from the Fayetteville, NC, plant where Nemours (DuPont’s successor) manufactures GenX is contaminated with fluorochemicals. Even after Nemours began to collect its wastewater for disposal in a deep injection well in Texas, perfluorinated ethers such as GenX are mysteriously being detected in the river.
MOTW Update: February 27, 2017
Perfluorooctanoic acid (PFOA) was the Molecule of the Week for August 10, 2015. The following September, DuPont, a long-time manufacturer of PFOA, went to trial for discharging the chemical into aquifers used for drinking water in West Virginia. That was the first of six bellwether suits of its kind. Earlier this month, DuPont announced that the Ohio multidistrict litigation, which involved leaks from the same West Virginia plant, had been settled.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

