What molecule am I?
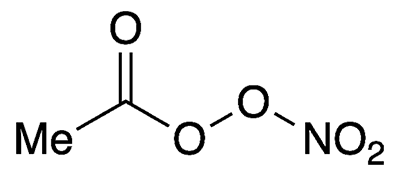
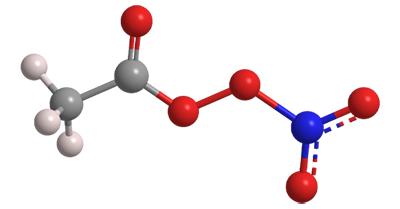
Peroxyacetyl nitrate is an unstable, highly oxygenated compound that exists only in the atmosphere. It is a key intermediate in the formation of the air pollutant ozone.
In December 2019, Emily V. Fischer at Colorado State University (Fort Collins) was awarded the James B. Macelwane Medal at the fall meeting of the American Geophysical Union. The medal is awarded for “significant contributions to the geophysical sciences by an outstanding early career scientist”. Fischer’s current work explores the processes that redistribute anthropogenic reactive nitrogen to remote regions.
In Fischer’s nomination citation, Ronald C. Cohen at the University of California, Berkeley, wrote
Tropospheric ozone is not emitted but made in situ from hydrocarbons, sunlight, and oxides of nitrogen. Nitrogen oxides are usually short-lived but can be carried to distant locations via the formation of the temporary reservoir species peroxyacetyl nitrate (PAN).
PAN spreads throughout the lower regions of the atmosphere and decomposes to nitrogen oxides, and thus plays a part in the formation of ozone. Ozone higher up in the atmosphere protects us from harmful cosmic radiation, but in the troposphere, it is a harmful pollutant.
Although PAN is a hazardous substance, specific hazard information for it is not given here because it is not an article of commerce. It is an inhalation toxin and eye irritant; and it is extremely explosive in the liquid state.
[Many thanks to Nancy McGuire for assistance with this article.—ED]
Peroxyacetyl nitrate
fast facts
| CAS Reg. No. | 2278-22-0 |
| SciFinder nomenclature | Peroxide, acetyl nitro |
| Empirical formula | C2H3NO5 |
| Molar mass | 121.05 g/mol |
| Appearance | Unstable liquid or gas |
| Boiling point | Decomposes upon heating |
| Water solubility | Decomposes |
MOTW update
Thiamethoxam, clothianidin, and imidacloprid were the Molecules of the Week for October 15, 2012, February 17, 2014, and August 25, 2014, respectively. They are nicotinoid pesticides that pose neurotoxic risks to humans and wildlife, especially honeybees. Nonetheless, in an interim decision announced in January, the US Environmental Protection Agency is allowing continued use of the pesticides, with some restrictions yet to be announced. This action is in contrast to the European Commission’s 2018 ban on these and other nicotinoids for outdoor use.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

