What molecules are we?

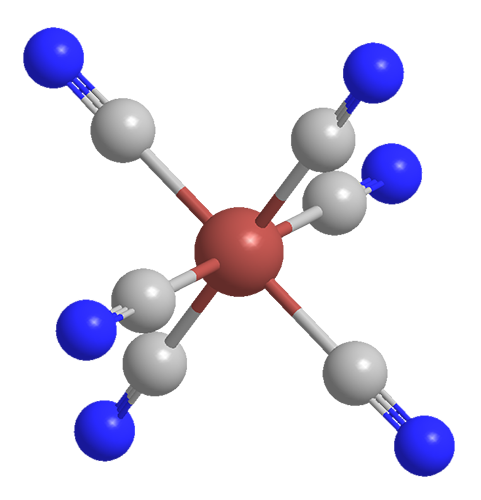
The pigment Prussian blue consists of iron cations, cyanide anions, and water. The empirical formula—minus the water of crystallization—is Fe7(CN)18. This seems odd with respect to the iron oxidation state until you learn that the complex contains Fe(II) and Fe(III). Thus, the formula that gives a truer idea of its composition is Fe4[Fe(CN)6]3. Its formal name is iron(III) hexacyanoferrate(II).
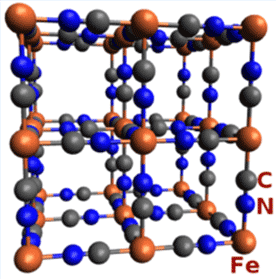
As shown in the two left-hand drawings, the Fe(CN)6 anion in Prussian blue is octahedral. The right-hand drawing shows its unit cell, which has a cubic lattice structure. In addition to having as many as 16 molecules of water per formula unit, the compound usually contains inorganic impurities, which can affect its color.
The name Prussian blue originated in the 18th century, when the compound was used to dye the uniform coats for the Prussian army. Over the years, the pigment acquired several other “blue” names, including Berlin, Parisian, and Turnbull’s blue. It has been used for centuries in unusually diverse applications (see the information box). Despite the presence of cyanide groups, the pigment is not toxic to humans.
Seniors among us will recognize “Prussian blue” as a crayon color. Prussian blue was one of the 38 original Crayola colors introduced by Binney & Smith Inc. in 1903. (The company name was subsequently changed to Crayola; later, the firm was acquired by Hallmark.)
The Prussian blue crayon name lasted until 1958, when it was changed to midnight blue. The reason for the change is unclear: One source says it was made because no one knew what Prussia was anymore; another reports that the move was spurred by political correctness during the Cold War.
| Paints, inks, and enamels |
| Textiles, rubber, and plastics |
| Antidote for heavy-metal poisoning |
| Histopathology stain for detecting iron |
Typewriter ribbons and carbon paper (now obsolete of course!) |
MOTW update:
April 26, 2021
Prussian blue is a pigment that is used to color paints, inks, textiles, and other commercial products. But during the past decade, Prussian blue found a high-tech use: Its ability to transfer electrons efficiently makes it an ideal substance for use in sodium-ion battery electrodes. Battery producer Natron Energy (Santa Clara, CA) recently concluded a deal with Lonza Group (Basel, Switzerland) to supply 700–1000 t/year of Prussian blue for the Natron BlueTray 4000 battery system to be used for data-center and telecommunications applications.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

