What molecule am I?
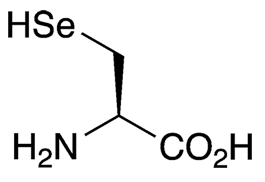
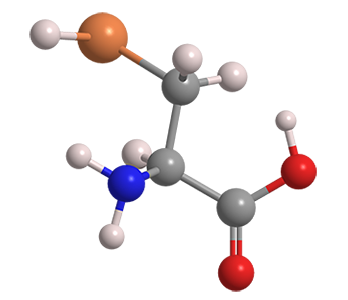
Selenocysteine is often called the 21st essential (protein-forming) amino acid. It is found in countless species of all domains of life. Much research has been reported on the biochemistry of selenocysteine in the North American plant Astragalus bisulcatus.
Scientists long believed that fungi were an exception to the broad prevalence of selenocysteine in life forms. But recently, Vadim N. Gladyshev, Marco Mariotti, and co-workers at Harvard Medical School (Boston) found selenocysteine-containing proteins in three fungus phyla. A few fungal genes that contain selenoproteins behave similarly to those in other organisms; but the mechanisms and purposes of other fungal “selenogenes” are as yet unknown.
Selenium’s wealth of natural and synthetic isotopes makes radiolabeled selenocysteine (Sec) a valuable tool in analytical chemistry and biochemistry. Specifically, 73Se-Sec is used in positron emission tomography (PET); 75Se-Sec in protein X-ray crystallography; and 77Se-Sec in high-resolution nuclear magnetic resonance (NMR) spectroscopy.
Selenocysteine hazard information
| GHS classification*: skin corrosion/irritation, category 2 | |
| H315—Causes skin irritation | |
| GHS classification: serious eye damage/eye irritation, category 2A | |
| H319—Causes serious eye irritation | |
| GHS classification: specific target organ toxicity, single exposure; respiratory tract irritation, category 3 | |
| H335—May cause respiratory irritation | |
*Globally Harmonized System of Classification and Labeling of Chemicals. Explanation of pictograms.
Selenocysteine fast facts
| CAS Reg. No. | 10236-58-5 |
| Empirical formula | C3H7NO2Se |
| Molar mass | 168.05 g/mol |
| Appearance | White to light yellow crystals or powder |
| Melting point | 143–146 ºC |
| Water solubility | ≈400 g/L |
MOTW update
Loratadine was the Molecule of the Week for July 10, 2006. It is a venerable nonsedating antihistamine that is widely used to combat environmental allergens. Recently, Heather B. Miller, Meghan S. Blackledge, and colleagues at High Point University (NC) discovered that loratadine causes some otherwise antibiotic-resistant bacteria (e.g., Staphylococcus epidermidis and S. aureus) to become susceptible to β-lactam antibiotics and vancomycin (the September 5, 2016 MOTW).

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

