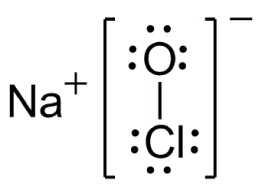
If you weren't a chemist, you'd know sodium hypochlorite (NaClO) as bleach, or possibly as the "chlorine" you put in your swimming pool or spa. NaClO solution is a convenient way to handle chlorine in an aqueous solution; it is prepared by absorbing chlorine gas in sodium hydroxide solution. Industrially, NaClO solution is produced directly by electrolyzing sodium chloride solution.
MOTW update:
August 9, 2021
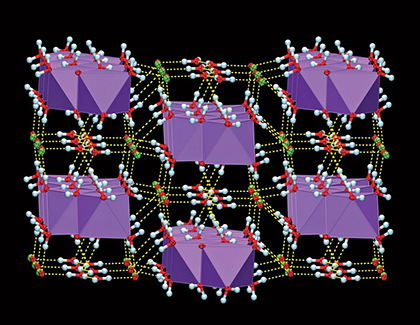
Sodium hypochlorite (NaClO) has been widely used for >200 years; but until now, the crystal structure of its pentahydrate (the normal solid form) had not been established. Tomislav Friščić and co-workers at McGill University (Montreal), working at –100 °C because NaClO•5H2O liquefies at ambient temperature, determined the structure of a single crystal, shown. The structure consists of alternating layers of Na+ and ClO– ions “glued” together by water molecules.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

