What molecule am I?
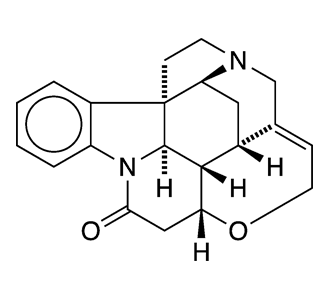
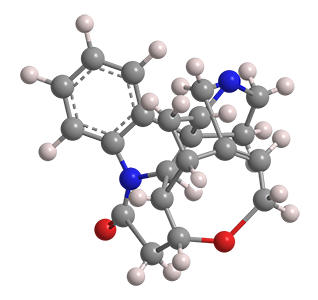
(–)-Strychnine, the natural form of the notorious toxin, was the Molecule of the Week for December 2, 2013. Robert B. Woodward’s 1954 classical total synthesis of the alkaloid helped him win the Nobel Prize in Chemistry for 1965.
(+)-Strychnine, the molecule’s non-natural enantiomer, presents, if anything, a more difficult synthesis. The feat was first accomplished in 1995 by Larry E. Overman’s research group at the University of California, Irvine. They claimed the first asymmetric total synthesis of (–)-strychnine and used a similar reaction sequence to produce the (+)-enantiomer.
Borrowing on techniques used in more recent syntheses of the (–) isomer, Yong Qin and co-workers at the West China School of Pharmacy (Chengdu) reported a more concise synthesis of (+)-strychnine in late 2018. Qin’s method required 13 reaction steps, including two cascade reactions: a photolytic radical cascade and a bioinspired oxidation–rearrangement.
(+)-Strychnine’s properties appear to have been studied very little, if at all. Of course, its fundamental physical and chemical properties should be the same as those of its enantiomer (see fast facts box). But whether its toxicology is similar to that of its mirror twin remains to be seen.
MOTW updates
Succinic acid was the Molecule of the Week for March 30, 2009. At the time, it was considered to be an important biobased chemical; and several projects were under way to produce it from agricultural byproducts. But this past week, the last biosuccinic acid joint venture was dissolved; and the plants built a decade ago to make the product are idle.
Dichloromethane (aka methylene chloride) was a very recent Molecule of the Week: March 4, 2019. It has been a useful sovlent for decades; but its significant hazards have caused it to be used in a declining number of applications. In mid-March, the US Environmental Protection Agency finalized a ban on dichloromethane as an ingredient in paint and coating removers used by consumers, effective at the end of 2019. Environmental and health activists say that the ban, originally promulgated during the Obama administration for consumer and industrial use, does not go far enough.
(+)-Strychnine fast facts
| CAS Reg. No. | 163956-38-5 |
| Empirical formula | C21H22N2O3 |
| Molar mass | 334.41 g/mol |
| Appearance | Translucent crystals or white powdera |
| Melting point | 268–286 ºCa,b |
| Water solubility | ≈200 mg/La |
aData for (–)-strychnine.
bDepends on heating rate.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

